To the Editor,
Synchronous and metachronous liver metastases from colorectal cancer (CRC) can be resected with curative intent, but are initially unresectable in about 80% of patients because of extrahepatic disease, involvement of non-resectable structures, or insufficient remaining liver tissue [Citation1]. In these patients, long-term survival is rare. Some patients with initially unresectable colorectal liver metastases (CLM) may become candidates for curative resection after tumor shrinkage by conversion chemotherapy [Citation2].
In 2005, Folprecht et al. [Citation3] reported that the resection rate in patients with liver-limited metastases strongly correlates with the objective response rate (ORR) to chemotherapy (r = 0.96; p = 0.002) on the basis of an analysis of five prospective trials and one retrospective study. Chemotherapy with monoclonal antibody (mAb) was not included in these six studies. At present, three targeted agents (bevacizumab, cetuximab, and panitumumab) improve outcomes when combined with first-line, systemic combination chemotherapy in patients with metastatic CRC. We therefore updated the analysis of Folprecht et al. [Citation3] and added data from clinical trials of systemic chemotherapy with mAb. The main objective of the data reanalysis was to explore the relationship between ORR and resection rate in patients with initially unresectable CLM.
We updated the data from the trials by Alberts et al. [Citation4] and Quenet et al. [Citation5] and excluded the data from the trial by Zelek et al. [Citation6] because hepatic arterial infusion was used. Data on first-line systemic chemotherapy with mAb were extracted from recently published reviews which discussed the role of chemotherapy in patients in conjunction with liver resection [Citation2,Citation7]. We then summarized the ORR and resection rate for R0 or R1 resection in patients with CLM alone in five trials without mAb and seven trials with mAb [Citation8–13] (). If the data were available, patients who received radiofrequency ablation were considered to have undergone R0 or R1 resection. Clearly, there was no trend toward a correlation between ORR and resection rate () (r = 0.045; p = 0.50). Moreover, the resection rate appears to have reached a plateau at an ORR of about 60%, with the exception of the data reported by Ychou et al. [Citation5].
Figure 1. Rate of liver resection after chemotherapy in patients with initially unresectable colorectal liver metastases alone. The solid dots and the circles represent chemotherapy with and without monoclonal antibodies, respectively.
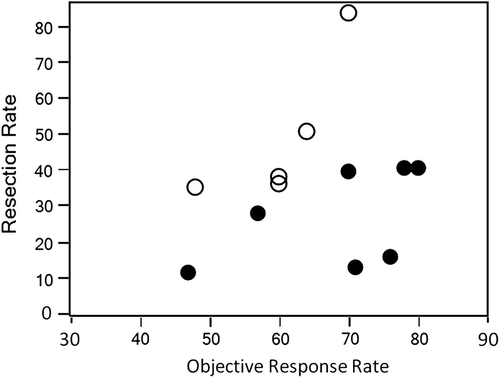
Table I. Effect of combination regimens in downsizing chemotherapy of colorectal liver metastases
Higher response rates are probably associated with more patients becoming candidates for resection in patients with CLM who were regarded as ‘initially unresectable’. However, our analysis does not support this strategy. One possible explanation might be that the ORR reached a plateau in several phase II and III clinical trials involving combination with mAb. Another explanation may be the different definitions of resectable CLM among clinical trials, which has significantly affected the analysis of data evaluating conversion chemotherapies. It is difficult to determine which patients with CLM are candidates for surgical resection, as the definition of resectability varies between surgeons and clinics. In particular, there is no generally agreed definition of ‘initially unresectable’ CLM. For instance, the difficulty in defining resectability was further highlighted in the CELIM study [Citation11] by examining the role of clinical judgment. Wide inter-individual variations in the decision-making process with regard to CLM resectability were observed and in 6.8% of cases when one surgeon classified a CLM as resectable, another surgeon completely disagreed. Patient selection with strict criteria for non-resectability may be also one of the reasons for the lack of further increase in resection rates.
Assessing the response on computed tomography may not be sufficient to measure the efficacy of a systemic treatment with chemotherapy and a mAb. Recent reports have indicated that the pathological response to a systemic treatment could become a relevant indicator of a patient's prognosis [Citation14]. The activity of bevacizumab is independent of KRAS status and, when used in combination with chemotherapy, has obtained a high rate of pathological complete responses, from 8% to 23% in recent reports [Citation14–16]. At present, no data are available on pathological response after the treatment of CLM with cetuximab or panitumumab. In the goal of conversion chemotherapy, key factors might be not only tumor shrinkage but pathological response, followed by prolongation of survival in patients with CLM.
The ORR thus reached a plateau with respect to directly increasing the resection rate in the era of combination chemotherapy with mAb. It is unlikely that the ORR and resection rate can be an adequate surrogate for overall outcome in patients with initially unresectable CLM in clinical trials developing conversion chemotherapies with mAb.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adam R. Chemotherapy and surgery: New perspectives on the treatment of unresectable liver metastases. Ann Oncol 2003;14(Suppl 2):ii13–6.
- Alberts SR. Update on the optimal management of patients with colorectal liver metastases. Crit Rev Oncol Hematol 2012;84:54–90.
- Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann Oncol 2005;16:1311–9.
- Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J Clin Oncol 2005;23:9243–9.
- Ychou M, Viret F, Kramar A, Desseigne F, Mitry E, Guimbaud R, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): A phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharmacol 2008;62:195–201.
- Zelek L, Bugat R, Cherqui D, Ganem G, Valleur P, Guimbaud R, et al. Multimodal therapy with intravenous biweekly leucovorin, 5-fluorouracil and irinotecan combined with hepatic arterial infusion pirarubicin in non-resectable hepatic metastases from colorectal cancer (a European Association for Research in Oncology trial). Ann Oncol 2003;14:1537–42.
- Quan D, Gallinger S, Nhan C, Auer RA, Biagi JJ, Fletcher GG, et al. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: A systematic review. Surgery 2012;151:860–70.
- Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, et al. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer 2009;101:1033–8.
- Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol 2011;22:2042–8.
- Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: A phase 2 trial. Lancet Oncol 2010;11:845–52.
- Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38–47.
- Bokemeyer C, Cutsem EV, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466–75.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol 2010;28:4697–705.
- Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008;26:5344–51.
- Klinger M, Tamandl D, Eipeldauer S, Hacker S, Herberger B, Kaczirek K, et al. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol 2010;17:2059–65.
- Bertolini F, Malavasi N, Scarabelli L, Fiocchi F, Bagni B, Del Giovane C, et al. FOLFOX6 and bevacizumab in non-optimally resectable liver metastases from colorectal cancer. Br J Cancer 2011;104:1079–84.
- Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol 2007;18:299–304.