Abstract
Background. Increased awareness of the adverse effects of cancer treatments has prompted the development of fertility preserving regimens for the growing population of cancer survivors who desire to have children of their own. Material and methods. We conducted a registry-based study to evaluate the risk of stillbirth, early death and neonatal morbidity among children of female cancer survivors (0–34 years at diagnosis) compared with children of female siblings. A total of 3501 and 16 908 children of female cancer patients and siblings, respectively, were linked to the national medical birth and cause-of-death registers. Results. The risk of stillbirth or early death was not significantly increased among offspring of cancer survivors as compared to offspring of siblings: the risk [Odds Ratio (OR)] of early neonatal death, i.e. mortality within the first week was 1.35, with a 95% confidence interval (CI) of 0.58–3.18, within 28 days 1.40, 95% CI 0.46–4.24 and within the first year of life 1.11, 95% CI 0.64–1.93 after adjustment for the main explanatory variables. All these risk estimates were reduced towards one after further adjustment for duration of pregnancy. Measures of serious neonatal morbidity were not significantly increased among the children of survivors. However, there was a significant increase in the monitoring of children of cancer survivors for neonatal conditions (OR 1.56, 95% CI 1.35–1.80), which persisted even after correcting for duration of pregnancy, that might be related to parental cancer and its treatment or increased surveillance among the children. Conclusion. Offspring of cancer survivors were more likely to require monitoring or care in a neonatal intensive care unit, but the risk of early death or stillbirth was not increased after adjustment for prematurity. Due to the rarity of the mortality outcomes studied, collaborative studies may be helpful in ruling out the possibility of an increased risk among offspring of cancer survivors.
Survivors of cancer who may be able to have children of their own are concerned about the health and well-being of their future offspring [Citation1,Citation2]. Increasing awareness of the adverse effects of cancer treatments on the reproductive system has led to the development of fertility preserving regimens, making parenthood possible for a growing population of early onset cancer survivors [Citation3]. Although little evidence exists to support an increased risk of genetic disease in the offspring [Citation4–7], risk of preterm delivery has been shown to be elevated among offspring of female cancer survivors [Citation8–10], related quantitatively in one study to high radiation doses to the immature uterus of young girls treated before menarche [Citation9]. Previous studies evaluating the risk of stillbirth among female cancer survivors did not find significant differences between former cancer survivors and the comparison group [Citation11,Citation12]. However, a report from the Childhood Cancer Survivor Study (CCSS) found a positive dose-response association between uterine and ovarian radiation and the risk of stillbirth and neonatal death among offspring of female survivors [Citation13], but this could not be confirmed by a recent Danish case-cohort study [Citation14].
Pregnancy outcomes previously studied in the population of former cancer survivors include low birth weight and preterm birth [Citation8,Citation9,Citation11,Citation15], stillbirths [Citation11], spontaneous [Citation12] and induced abortions [Citation16]. Most previous studies are limited to the subgroup of childhood cancer survivors [Citation9,Citation10]. One study reported overall hospitalizations of offspring as well as hospitalizations due to perinatal causes to be similar among survivor and sibling offspring [Citation17]. Although the risk of certain obstetric complications such as preterm delivery is well-established, neonatal health and mortality of offspring have been infrequently studied. As prematurity is a major cause of neonatal morbidity and mortality in developed countries and because the offspring of cancer survivors have in general an elevated risk of prematurity, it is important to evaluate the risk of mortality and morbidity in the children of cancer survivors. Furthermore, stillbirths and early deaths within the neonatal period have been considered measures of genetic disease in the progeny of cancer survivors.
In a population-based approach, using nationwide health registries, we explored stillbirth, neonatal and infant death and neonatal morbidity among offspring of female cancer survivors treated in childhood, adolescence or early adulthood (aged below 35 years at diagnosis), compared with a cohort of offspring of female siblings of former early onset cancer patients.
Material and methods
In Finland, a unique personal identity code (PIC) has been given to each citizen and permanent resident since 1964–1967. This code allows for individual linkage of records from different registries and databases. Identification of the cancer patient, sibling, and offspring cohorts, as well as data on the endpoints studied were obtained using four nation-wide registers: the Finnish Cancer Registry, the Central Population Register (CPR), the Medical Birth Register, and the Cause-of-Death Register.
Registers used in the study
The Finnish Cancer Registry began systematic registration of cancer cases in 1953. The registry is population-based, nation-wide and almost 100% complete (100% for solid tumors, over 90% for hematological malignancies, and 100% for childhood cancers) [Citation18]. Data recorded in the registry include the PIC, details of primary cancer site and histology, stage, and date of diagnosis.
The CPR was founded in 1969. It is nation-wide and includes the name and former names, PIC, municipality of birth and residence, the date of emigration or date of death if applicable of each individual living in Finland and alive in 1967 or born thereafter. Within the CPR, individuals born in 1955 or thereafter can be linked reliably to their parents, siblings and offspring. Siblings of cancer patients were identified from the CPR by linking the PIC of the patient to his or her mother. All offspring of patients and siblings were also identified from the CPR by maternal link.
The Medical Birth Register began in 1987 and contains individual data on all mothers giving birth and on all children born in Finland. The data include identification codes both for mothers and children. All live births and stillbirths at a birth weight of at least 500 g or a gestational age of at least 22 weeks are included in the registry. The data are received from hospitals or from the midwife or physician assisting in the delivery. Data for less than 0.1 per cent of infants are missing in the Medical Birth Register during the study period [Citation19].
Statistics Finland maintains the Cause-of-Death Register for all deaths in Finland. The data are compiled from death certificates and supplemented with data from the CPR. Death certificates are checked by physicians regionally and centrally at Statistics Finland. Data on causes of death have been available in computerized form since 1969.
Study cohorts
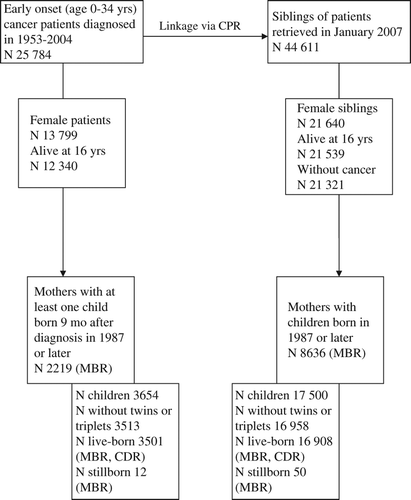
A total of 25 784 males and females between the ages 0 and 34 years were diagnosed with cancer between 1953 and 2004 and identified within the Finnish Cancer Registry. The personal identity code of the cancer patients was used for linkage to the CPR and 44 611 full and half siblings of these patients were identified (). Likewise, live-born offspring of both cancer survivors and siblings were identified from the CPR.
Figure 1. Cohorts of former cancer patients, siblings of cancer patients and offspring. Description of linkage with the Cause- of-Death Register (1969–2007) and Medical Birth Register (1987–2008) to obtain information on early deaths and stillbirths, respectively. CDR, Cause-of-Death Register; MBR, Medical Birth Registry; mo, months; yrs, years.
Offspring of cancer survivors and siblings
There were 13 799 female cancer survivors under the age of 35 years at diagnosis of whom 12 340 attained reproductive age (16 years) (). From the Medical Birth Register, we identified 2219 patients with post-diagnosis deliveries since 1987 and 3654 still- or live-born offspring born at least nine months after diagnosis. Of these, 3513 were singletons and included in the analyses of stillbirths and neonatal outcomes.
In all, 44 611 siblings of cancer patients were identified by linkage to the CPR. Of these 21 640 were females of whom 21 539 attained reproductive age. After excluding 218 female siblings with early onset cancer (included in the survivors), we were left with 21 321 healthy female siblings who could be linked to the Medical Birth Register. From the Medical Birth Register, information on the deliveries of 8636 siblings and their 17 500 offspring born after 1987 were identified. A total of 16 958 singleton offspring were included in the analyses of stillbirths and neonatal outcomes (). Deaths were analyzed for offspring born in 1987 or thereafter.
Outcomes
The offspring of female cancer survivors and offspring of female siblings were linked to the Cause-of-Death Register to obtain the dates and causes of death from 1987 to 2008. Early neonatal mortality was defined as a death occurring during the first week of life (0–6 days), neonatal mortality was defined as a death occurring within the first four weeks of life (0–27 days), and infant mortality as any death occurring during the first year of life (0–364 days). We also explored perinatal mortality by combining stillbirths and early neonatal deaths into one outcome category. The cause of death was studied in the following groups: deaths from disease and medical conditions [including sudden infant death syndrome (SIDS)] and deaths from external causes and poisonings, or from unintentional and intentional accidents and injuries. Causes of death related to diseases and medical conditions were grouped in the following categories: congenital anomalies and conditions, prematurity and delivery complications, infections and malignancy.
Female cancer survivors and female siblings were also linked to the Medical Birth Register to obtain information on stillbirths and various adverse neonatal outcomes of their children up to the end of 2008. Stillbirth in Finland is defined as a fetus that was not viable at birth at a minimum of 22 weeks of gestation and/or weighing at least 500 g at birth. Neonatal cardiopulmonary distress was defined as a need for cardiopulmonary resuscitation or ventilation assistance. Asphyxia was defined as a one minute Apgar score lower than 7 and artery pH lower than 7, or solely an artery pH lower than 7 if the Apgar score was missing. In the MBR, information on five minutes Apgar scores were not collected between October 1990 and December 2003, and therefore not used in our study. The need for monitoring of the infant was defined as a transfer to a neonatal intensive care unit, an observation ward, or transfer to another hospital or another ward within the same hospital.
Statistical analysis
Multiple logistic regression models were used to calculate odds ratios (ORs) for adverse outcomes for singleton births. Models exploring neonatal mortality were adjusted for the mother's previous history of a neonatal death, infant sex, maternal age, calendar time (by decade), and birth order (based on the mother's pervious deliveries). To account for the effect of preterm delivery, a separate model further adjusting for duration of pregnancy was applied. Models for stillbirth and neonatal morbidity outcomes included the following basic set of explanatory variables: infant sex, birth order, duration of pregnancy in full weeks, year of delivery, maternal age, maternal hypertension, maternal smoking, pre- eclampsia, maternal infections, gestational diabetes or impaired glucose tolerance, placental problems (including premature detachment and placenta pre- via). Maternal age was treated as a categorical variable with the following three categories: 1) less than 20 years; 2) 20–34 years and 3) 35 years or more. Birth order and year of delivery were treated as continuous variables, while all other variables were dichotomous.
To evaluate the possible effects of age at cancer diagnosis on the outcomes, patients were further divided into three diagnostic age groups as follows: childhood survivors (0–14 years at diagnosis), adolescent survivors (15–19 years at diagnosis), and young adult survivors (20–34 years at diagnosis). Multiple pregnancies of the same mother were included in the analyses using random effects modeling to take into account the dependent nature of the data of children born to the same woman. All the results presented have been calculated using these models. The log-likelihood ratio test was performed in the process of choosing models and variables for the final analysis. Stata 11 software was used for all calculations (STATA Corp, College Station, Texas, USA).
Results
In total, there were 16 deaths occurring within the first year of life among offspring of female cancer survivors compared with 72 among offspring of siblings. In both groups the majority of deaths occurred within the first seven days of life 12/16 (75%) and 45/72 (63%), for cancer survivors and siblings offspring, respectively. There were 12 stillbirths among cancer survivors and 50 among siblings.
Overall, after adjusting for the major explanatory variables, cancer survivors did not have a significantly elevated risk of early death up to the age of one year (OR 1.11, 95% CI 0.64–1.93) compared with offspring of siblings (). The suggestion of an increased risk for early neonatal (first week of life) and neonatal (first month of life) death (OR 1.35, 95% CI 0.58–3.18 and OR 1.40, 95% CI 0.46–4.24) was explained in large part by prematurity as risks were decreased after adjustment for duration of pregnancy (OR 1.06 95% CI 0.50–2.25 and OR 1.07, 95% CI 0.53–2.15). No significant increase in risk of early deaths was observed in subgroup analyses by age at diagnosis (). ORs were also calculated for perinatal mortality (combining stillbirths and early neonatal deaths), but no significant elevation in risk could be observed after adjustments (OR 1.28, 95% CI 0.81–2.04). Further adjustment for duration of pregnancy reduced the OR to 1.00 (95% CI 0.62–1.62).
Table I. Risk of early neonatal death (within one week), neonatal death (within one month, first week included), and infant death (up to one year) from the Cause-of-Death registry (1987–2007) among offspring of female cancer survivors born nine months after diagnosis or later as compared with offspring of female siblings.
Table II. Risk of early neonatal (within one week), neonatal (within one month, first week included) and infant death (up to one year) from the Cause-of-Death Registry data (1987–2007) by age at diagnosis of survivor parent comparing offspring of female patients with offspring of female siblings.
In all, deaths from diseases and medical causes within the first year of life were similar among offspring of survivors and siblings. The largest categories of early death causes were delivery complications and prematurity as well as congenital anomalies and conditions. Among offspring of cancer survivors all 16 early deaths were due to medical causes, while the corresponding number among offspring of siblings was 65. Of deaths due to medical causes, among offspring of cancer survivors eight deaths (0.23%) were due to prematurity or delivery complication and eight (0.23%) due to congenital anomaly and disease while the corresponding numbers in the sibling offspring cohort were 31 (0.18%) and 30 (0.17%), respectively. Among the offspring of siblings seven (0.04%) were accidental or due to sudden infant death syndrome while no deaths were due to violent causes in offspring of cancer survivors.
Of the 12 stillbirths among survivors, one occurred in a childhood leukemia survivor, one in a survivor of colon adenocarcinoma in adolescence, and 10 in young adult survivors (five thyroid cancers, one central nervous system cancer, one Hodgkin lymphoma, one breast cancer, one cervix cancer, and one colon cancer). The risk of stillbirth and birth asphyxia was not elevated in offspring of female cancer survivors compared with offspring of siblings but there was a significantly increased risk (OR 1.90, 95% CI 1.65–2.19) for the need for intensive care or neonatal monitoring (including transfer to another hospital or ward), persisting after adjustment for duration of pregnancy among other explanatory variables (OR 1.44, 95% CI 1.25–1.66) (). This elevated risk was observed in all diagnostic age-groups compared with offspring of siblings (). The proportion of offspring requiring neonatal intensive care was 121/3501 (3.5%) and 428/16 908 (2.5%), in female cancer survivors and siblings, respectively. The proportion of offspring transferred to another hospital/ward was 112/3501 (3.2%) in offspring of female cancer survivors and 292/16 908 (1.7%) in the offspring of siblings. Though the risk for cardiopulmonary resuscitation or respirator care appeared significantly elevated in crude analyses, the associations disappeared after adjustment for duration of pregnancy.
Table III. Risk of stillbirth and various indicators of neonatal outcomes (cardio-pulmonary resuscitation or respirator care, monitoring or neonatal intensive unit care, and birth asphyxia) among offspring of women with a history of cancer as compared with offspring of female siblings using data from the Medical Birth Registry (1987–2008).
Table IV. Risk of stillbirth and various indicators of neonatal outcomes (cardio-pulmonary resuscitation or respirator care, monitoring or neonatal intensive unit care, and birth asphyxia) among offspring of women with a history of cancer grouped by age at diagnosis as compared with offspring of female siblings using data from the Medical Birth Registry (1987–2008).
Discussion
We studied early mortality (up to one year of age), stillbirth, and neonatal morbidity among offspring of female cancer survivors as compared with offspring of female siblings of former early onset cancer patients. The strengths of our study include the population-based approach which allowed complete identification of female cancer survivors, siblings and offspring, and the endpoints studied, yielding the largest offspring cohort to date exploring these outcomes. Furthermore, registry linkage allowed identification of the comparison group of siblings of former cancer patients. By including multiple deliveries per subject, we were able to explore the risk of the outcomes in all post-diagnosis offspring. Likewise, based on central population registration, the previous obstetric history could be taken into account by adjusting for background factors, such as birth order. Registry data provided information on outcomes studied and important confounders for cancer survivors and siblings alike. In this way the data, as opposed to self-reported data, were free of recall bias. Twin and triplet deliveries were excluded from the analyses, as they are known to be associated with the outcomes studied. Previous studies on mortality risk estimates are limited to the neonatal period [Citation11,Citation13,Citation14], and our study is, to the best of our knowledge, the first study reporting later deaths up to one year of life among offspring of cancer survivors.
Limitations of our study include, despite the use of population-based nationwide data, the relatively small number of stillbirths and neonatal deaths occurring in children of female cancer survivors and siblings, and the lack of detailed information on type of cancer treatments received (e.g. doses of radiotherapy or chemotherapy, details of surgery) which precluded dose-response evaluations.
Our results do not rule out the possibility of an elevated risk of death during the neonatal period among the offspring of the 2219 former cancer patients in comparison with offspring of 8636 female siblings. However, the suggestion in our data of an increased risk of death during the first week and month of life appeared to be attributable to the previously reported higher incidence of preterm birth among offspring of cancer patients [Citation8,Citation9,Citation11,Citation15].
One previous study [Citation13] did not report overall risk of neonatal death, but rather explored neonatal death in relation to cancer treatment received by 1627 mothers and found neonatal death to be elevated among offspring of cancer patients who had received high doses of uterine or ovarian irradiation (> 10 Gy) and a dose-response relationship was found. Another study of 917 female cancer survivors explored overall risk of neonatal death and reported a non-significantly increased risk in survivors offspring compared to offspring of healthy population controls (OR 1.37, 95% CI 0.42, 4.45) [Citation11].
Our data indicated no significant difference in the risk of stillbirth in offspring of cancer survivors compared with offspring of siblings. Previous studies were similarly unable to demonstrate a significant difference between exposed offspring and healthy controls [Citation11,Citation12].
Results concerning dose-response relationships between gonadal irradiation and stillbirth are contradictory. A report from the Childhood Cancer Survivor Study (CCSS) found a positive dose-response association between uterine and ovarian radiation and the risk of stillbirth and neonatal death among offspring of female survivors [Citation13]. Another report from a Danish study exploring dose-response relationships between cancer treatments and stillbirths and neonatal death, found no association between radiotherapy doses to the gonad and these untoward pregnancy outcomes [Citation14].
Different definitions of stillbirth may explain variability between results to some extent. The lack of treatment data in our study limited comparability to these results.
Etiology of stillbirth is multifactorial [Citation20,Citation21]. According to some estimates, in one quarter of cases the underlying etiology is genetic [Citation22], consisting mainly of karyotypic abnormalities, though single gene disorders and sporadic multiple malformation syndromes may also result in stillbirth. Cord complications and placental etiologies have been implicated in one of four cases. Other maternal risk factors include pre-eclampsia, maternal infection, nulliparity, smoking and high maternal age [Citation23]. In our survivor cohort, none of the women who delivered stillborn infants suffered from pre-eclampsia or were diagnosed with placental ablation or placenta previa. Two infants were in breech presentation and were delivered by cesarean section. As we did not have information on karyotype or autopsy findings in these infants, we cannot rule out the possibility that the stillbirth outcome reflects genetic etiologies in our data.
The hypothesis of an increased risk of stillbirth and neonatal death among former cancer survivors is plausible for more than one reason. It has been shown that offspring of female cancer survivors are at increased risk of preterm birth [Citation8,Citation9,Citation11,Citation15]. As prematurity is a major cause of neonatal mortality and morbidity in developed countries, the offspring of cancer survivors may be expected to be at elevated risk for these adverse outcomes. Exposure to chemotherapy and radiotherapy has been hypothesized to cause chromosomal abnormalities and genetic disease. Stillbirths and early deaths within the neonatal period have been considered measures of genetic disease in the progeny of cancer survivors.
In our study, neonatal monitoring of the infant was significantly more likely to occur among offspring of cancer survivors, even after adjustment for duration of pregnancy. At least some of the elevation was due to a higher proportion of offspring of survivors being transferred to another hospital or ward compared with offspring of siblings. According to our data, the proportion of offspring being admitted to a neonatal intensive care unit was higher (3.5% vs. 2.5%) among cancer survivors than among siblings. Maternal history of cancer may place these infants under closer observation. There is, thus, a possibility of a surveillance bias as healthcare professionals may express concern for the health of a child of a cancer survivor. It is also possible that the infants of patients remain in the hospital due to maternal postnatal care. Clark et al. [Citation11] explored admittance to a neonatal intensive care unit as one outcome and did not find the risk to be elevated for offspring of cancer survivors in Scotland.
We did not find the risk of birth asphyxia to be elevated among offspring of cancer survivors nor were they more likely to need cardiopulmonary resuscitation or ventilation assistance in comparison with offspring of siblings after adjustment for duration of pregnancy. The observed elevation in the crude analysis was due to a higher proportion of premature infants. These results are in accordance with a study on hospitalizations of offspring of cancer survivors, which found the risk of hospitalization due to perinatal causes (including asphyxia and respiratory distress) to be similar among offspring of survivors and siblings [Citation17].
We studied early mortality (up to one year of age), stillbirth, and neonatal morbidity among offspring of female cancer survivors as compared with offspring of female siblings of former patients in a population-based setting using registry data. Our data indicated no significant difference in the risk of stillbirth or early death in offspring of cancer survivors compared with offspring of siblings. However, offspring of cancer survivors appeared more likely to require monitoring or care in a neonatal intensive care unit, which is not solely accounted for by prematurity. Due to the rarity of infant death, further collaborative studies may be helpful in ruling out the possibility of an increased risk of stillbirth or neonatal mortality among offspring of cancer survivors.
Declaration of interest: The authors report no conflicts of interest. The article's contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding sources.
This study was supported by a grant from the National Cancer Institute (Grant number R01 CA104666). Funding was also provided by the Finnish Cancer Organizations and the Orion-Farmos Research Foundation.
References
- Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer 1999;86:697–709.
- Reinmuth S, Liebeskind AK, Wickmann L, Bockelbrink A, Keil T, Henze G, et al. Having children after surviving cancer in childhood or adolescence – results of a Berlin survey. Klin Padiatr 2008;220:159–65.
- Madanat LM, Malila N, Dyba T, Hakulinen T, Sankila R, Boice JD, Jr., et al.Probability of parenthood after early onset cancer: A population-based study. Int J Cancer 2008;123: 2891–8.
- Byrne J, Rasmussen SA, Steinhorn SC, Connelly RR, Myers MH, Lynch CF, et al. Genetic disease in offspring of long-term survivors of childhood and adolescent cancer. Am J Hum Genet 1998;62:45–52.
- Reulen RC, Zeegers MP, Lancashire ER, Winter DL, Hawkins MM. Offspring sex ratio and gonadal irradiation in the British Childhood Cancer Survivor Study. Br J Cancer 2007;96:1439–41.
- Green DM, Fiorello A, Zevon MA, Hall B, Seigelstein N. Birth defects and childhood cancer in offspring of survivors of childhood cancer. Arch Pediatr Adolesc Med 1997;151: 379–83.
- Winther JF, Boice JD, Jr., Mulvihill JJ, Stovall M, Frederiksen K, Tawn EJ, et al. Chromosomal abnormalities among offspring of childhood-cancer survivors in Denmark: A population-based study. Am J Hum Genet 2004;74:1282–5.
- Madanat-Harjuoja LM, Malila N, Lahteenmaki PM, Boice JD, Jr., Gissler M, Dyba T. Preterm delivery among female survivors of childhood, adolescent and young adulthood cancer. Int J Cancer 2010;127:1669–79.
- Signorello LB, Cohen SS, Bosetti C, Stovall M, Kasper CE, Weathers RE, et al. Female survivors of childhood cancer: Preterm birth and low birth weight among their children. J Natl Cancer Inst 2006;98:1453–61.
- Reulen RC, Zeegers MP, Wallace WH, Frobisher C, Taylor AJ, Lancashire ER, et al. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev 2009;18:2239–47.
- Clark H, Kurinczuk JJ, Lee AJ, Bhattacharya S. Obstetric outcomes in cancer survivors. Obstet Gynecol 2007;110:849–54.
- Winther JF, Boice JD, Jr., Svendsen AL, Frederiksen K, Stovall M, Olsen JH. Spontaneous abortion in a Danish population-based cohort of childhood cancer survivors. J Clin Oncol 2008;26:4340–6.
- Signorello LB, Mulvihill JJ, Green DM, Munro HM, Stovall M, Weathers RE, et al. Stillbirth and neonatal death in relation to radiation exposure before conception: a retrospective cohort study. Lancet 2010;376:624–30.
- Winther JF, Olsen JH, Wu H, Shyr Y, Mulvihill JJ, Stovall M, et al. Genetic disease in the children of Danish survivors of childhood and adolescent cancer. J Clin Oncol 2012;30:27–33.
- Fossa SD, Magelssen H, Melve K, Jacobsen AB, Langmark F, Skjaerven R. Parenthood in survivors after adulthood cancer and perinatal health in their offspring: a preliminary report. J Natl Cancer Inst Monogr 2005:77–82.
- Winther JF, Boice JD, Jr., Svendsen AL, Frederiksen K, Olsen JH. Induced abortions in Danish cancer survivors: A population-based cohort study. J Natl Cancer Inst 2009; 101:687–9.
- Winther JF, Boice JD, Jr., Christensen J, Frederiksen K, Mulvihill JJ, Stovall M, et al. Hospitalizations among children of survivors of childhood and adolescent cancer: A population-based cohort study. Int J Cancer 2010;127: 2879–87.
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol 1994;33:365–9.
- Gissler M, Shelley J. Quality of data on subsequent events in a routine Medical Birth Register. Med Inform Internet Med 2002;27:33–8.
- Walsh CA, Vallerie AM, Baxi LV. Etiology of stillbirth at term: A 10-year cohort study. J Matern Fetal Neonatal Med 2008;21:493–501.
- Fretts R. Stillbirth epidemiology, risk factors, and opportunities for stillbirth prevention. Clin Obstet Gynecol 2010;53: 588–96.
- Wapner RJ. Genetics of stillbirth. Clin Obstet Gynecol 2010;53:628–34.
- Smith GC. Predicting antepartum stillbirth. Clin Obstet Gynecol 2010;53:597–606.
