To the Editor,
In general, radiotherapy (RT) is not considered the definitive treatment option for large liver tumors, including hepatoblastoma, because the liver cannot tolerate high-dose irradiation of a large volume. Meanwhile, proton beam therapy (PBT) is a particle RT that is capable of excellent dose localization because of the sharp and narrow dose peak, known as the Bragg peak. Therefore, PBT can be used to preserve more hepatic parenchyma, and liver tumors can be safely treated with proton beams, even with portal vein thrombosis [Citation1–3]. In 1996, we performed definitive PBT for a child with advanced hepatoblastoma who could not be managed with chemotherapy, surgery or liver transplantation. This patient was still doing well in 2012. Herein, we present the clinical course of this patient.
Case presentation
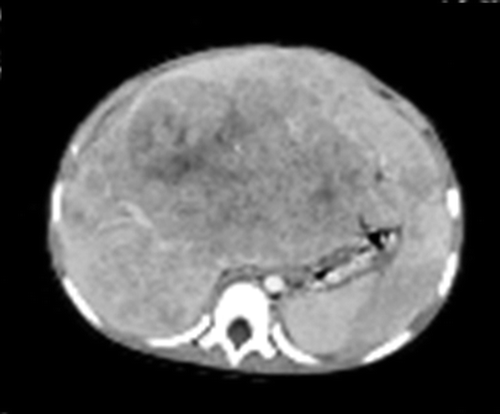
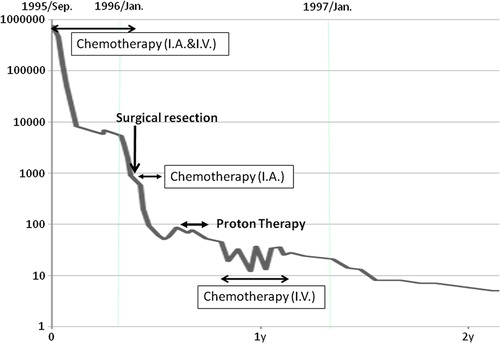
A two-year-old girl initially presented at another hospital with abdominal distention, and ultrasonography of her liver showed tumors in the liver. She was referred to our hospital in September 1995. An imaging study with MRI, CT and ultrasonography showed a large liver tumor (8 × 10 cm) in the left lobe invading to the right anterior and posterior segments with multiple intrahepatic metastases (). The serum alpha-fetoprotein (AFP) was 758 960 ng/ml. The patient was diagnosed with stage III and Pre-Treatment Extent of Disease (PRETEXT) IV hepatoblastoma. Monthly chemotherapy with cisplatin (CDDP) and doxorubicin (ADR) was immediately started via catheters in the right hepatic and celiac arteries in combination with intravenous ADR infusion [Citation4]. The AFP decreased rapidly to 3288 ng/ml after the third course of chemotherapy, but increased to 5811 and 6911 ng/ml before and after the fourth chemotherapy course, respectively. The treatment course and serum AFP levels are shown in . After the fifth course of chemotherapy was administered in January 1996, the AFP was 3700 ng/ml. The main tumor had shrunk (3 × 4 cm) and daughter lesions had virtually disappeared. However, it was considered that the tumor was developing resistance to the chemotherapy because the reduction of the AFP level was diminished, and that it would be difficult to administer another catheter injection. Therefore, extensive resection of the left lobe was attempted in February 1996, but this was not possible because the tumor thrombi were located within the middle and right hepatic vein, and also involved the right portal vein. Resection of the remaining daughter lesions and cholecystectomy were performed, and an arterial injection port was placed in the common hepatic artery. She received three courses of chemotherapy with CDDP and ADR via the injection port from February to March 1996. The AFP level initially decreased to approximately 50 ng/ml at the beginning of April, but slowly rose to 87 ng/ml by the end of April. The remaining main tumor was localized but was considered to be viable. Therefore, PBT was considered the optimal treatment in this situation and was attempted after obtaining informed consent.
The treatment dose of 51.15 GyE in 11 fractions (10.5 Gy in 3 fractions plus 36 Gy in 8 fractions) was applied over the course of 30 days, followed by three courses of adjuvant intravenous chemotherapy with CDDP, ADR, Etoposide (VP-16), and cyclophosphamide (CPM) (). The relative biological effectiveness (RBE) of the PBT was assumed to be 1.1 [Citation5,Citation6]. The proton beams (250 MeV) were generated by a booster synchrotron at the High Energy Accelerator Research Organization. Treatment rooms were equipped with either horizontal or vertical beams. The beam time allocated for the PBT was four hours per day, 120 days per year, in three separate intervals of 9–10 weeks. During PBT, the patient was sedated with pentazocine hydrochloride and hydroxyzine pamoate and immobilized in a styrofoam box that had been custom fitted to her.
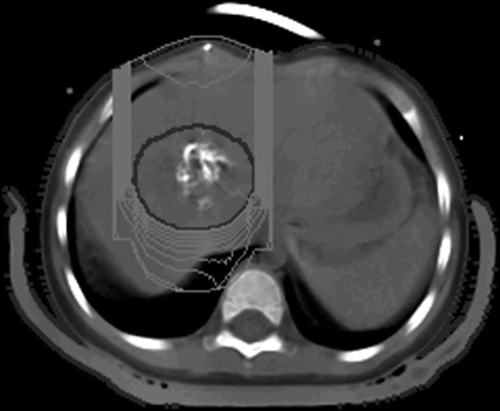
Figure 3. Treatment plan for proton beam therapy. The anterior beam was delivered to the main calcified tumor.
The serum AFP decreased gradually and a tumor biopsy performed four months after the PBT showed no malignancy. She experienced grade 2 duodenal ulcer, five months after the start of PBT, which was cured within one week by an oral histamine two receptor blocker.
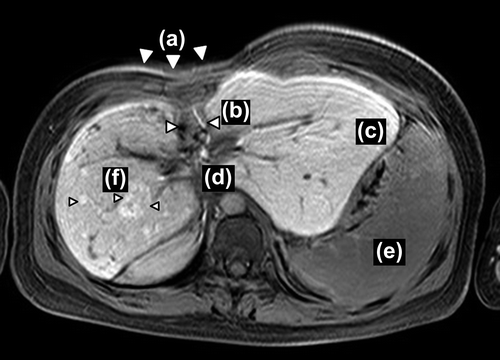
In 2012, 16 years after the PBT, the patient visited a general practitioner because of occasional nausea that mainly occurred after eating. Since abnormal liver function was detected by blood test, she was referred to our hospital. She has done well in her life as a student. Her skin was atrophic and the rib cage was depressed in the area that had been irradiated. MRI showed no malignancy in the liver, but did show compensatory hypertrophy of the left lateral lobe, AV-shunt of the posterior and anterior branch at the right lobe, portal vein stenosis with splenomegaly and multiple focal nodular hyperplasia (FNH)-like lesions scattered throughout the liver. The reason for the nausea was considered to be compression of the stomach as a result of the compensatory hepatic hypertrophy and splenomegaly ().
Discussion
At present, liver transplantation has been established as the definitive and curative treatment for unresectable hepatoblastoma [Citation7,Citation8]. Recent improvements in liver transplantation have resulted in better treatment outcomes with reduced mortality [Citation9–12]. The United Network of Organ Sharing reported that the 10-year survival of patients with hepatoblastoma after liver transplantation was 66% [Citation11]. However, when the current patient was admitted to our hospital in 1995, liver transplantation was not an accepted treatment modality in pediatric hepatic malignancy, particularly when it involved major hepatic vessels. Therefore, it was not possible for this patient to undergo liver transplantation at that time in Japan.
It is well known that the indication of curative RT for hepatoblastoma is limited, because it is difficult to maintain an adequate liver volume when tumors are irradiated at higher doses. Habland et al. treated hepatoblastomas with a median dose of 40 Gy (25–45 Gy), but only one of the four cases of unresectable hepatoblastoma was controlled with photon RT [Citation13].
There has been no report of PBT for hepatoblastoma, and PBT was very challenging for this patient. However, considering that PBT can deliver a high dose to the limited volume of the liver tumor, while delivering very low doses to non- cancerous liver tissue, and allowing preservation of a larger volume of normal liver tissue compared with photon RT, we believe that PBT was reasonable for this patient, even though the major vessels were inevitably located in the PBT field.
There have been some rare reports of patients with unresectable hepatoblastoma that were treated with chemotherapy alone [Citation14,Citation15]; however, as these are very uncertain cases, chemotherapy alone is not deemed to be sufficient. Furthermore, serum AFP levels decreased to normal levels during, or soon after, chemotherapy in these cases. In contrast, chemotherapy was not considered to be curative in our case based upon the changes in serum AFP.
Sixteen years later, while our patient was completely cured various long-term effects have now become apparent. To our knowledge, this is the first report showing late changes of the liver after irradiation during infancy. These late toxicities were not due to PBT alone; the FNH-like lesions and A-V shunt were due to chronic liver injury by the intra-arterial chemotherapy and PBT, and the compensatory hypertrophy occurred partly due to the surgical resection. Fortunately, severe subjective symptoms did not occur, but it is important to recognize and physicians should be informed about potential toxicities due to treatment, and the need for follow-up over the long-term.
In our case, PBT was able to be performed because the tumor had shrunk sufficiently to preserve enough hepatic parenchyma, even though vital hepatic vessels were involved with the tumor. PBT for hepatoblastoma was unprecedented, and should not be recommended as the first treatment choice, but may be useful for some patients with unresectable hepatoblastoma who are not suitable for complete resection or liver transplantation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was partially supported by the “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)”, initiated by the Council for Science and Technology Policy (CSTP).
References
- Mizumoto M, Tokuuye K, Sugahara S, Nakayama H, Fukumitsu N, Ohara K, . Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys 2008;71:462–7.
- Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, . Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol 2009;185:782–8.
- Hata M, Tokuuye K, Sugahara S, Kagei K, Igaki H, Hashimoto T, . Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer 2005;104:794–801.
- Sasaki F, Matsunaga T, Iwafuchi M, Hayashi Y, Ohkawa H, Ohira M, . Outcome of hepatoblastoma treated with the jplt-1 (Japanese study group for pediatric liver tumor) protocol-1: A report from the Japanese study group for pediatric liver tumor. J Pediatr Surg 2002;37:851–6.
- Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, . Relative biological effectiveness (rbe) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407–21.
- Gerweck LE, Kozin SV.Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol 1999; 50:135–42.
- Otte JB, de Ville de Goyet J. The contribution of transplantation to the treatment of liver tumors in children. Semin Pediatr Surg 2005;14:233–8.
- Tiao GM, Bobey N, Allen S, Nieves N, Alonso M, Bucuvalas J, . The current management of hepatoblastoma: A combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr 2005;146:204–11.
- Casas-Melley AT, Malatack J, Consolini D, Mann K, Raab C, Flynn L, . Successful liver transplant for unresectable hepatoblastoma. J Pediatr Surg 2007;42:184–7.
- Pimpalwar AP, Sharif K, Ramani P, Stevens M, Grundy R, Morland B, . Strategy for hepatoblastoma management: Transplant versus nontransplant surgery. J Pediatr Surg 2002;37:240–5.
- Austin MT, Leys CM, Feurer ID, Lovvorn HN, 3rd, O’Neill JA, Jr., Pinson CW, . Liver transplantation for childhood hepatic malignancy: A review of the united network for organ sharing (unos) database. J Pediatr Surg 2006; 41:182–6.
- Ismail H, Broniszczak D, Kalicinski P, Dembowska-Baginska B, Perek D, Teisseyre J, . Changing treatment and outcome of children with hepatoblastoma: Analysis of a single center experience over the last 20 years. J Pediatr Surg 2012;47:1331–9.
- Habrand JL, Nehme D, Kalifa C, Gauthier F, Gruner M, Sarrazin D, . Is there a place for radiation therapy in the management of hepatoblastomas and hepatocellular carcinomas in children? Int J Radiat Oncol Biol Phys 1992;23:525–31.
- Rana AN, Qidwai A, Pritchard J, Ashraf MS. Successful treatment of multifocal unresectable hepatoblastoma with chemotherapy only. Pediatr Hematol Oncol 2006;23: 153–8.
- Yokomori K, Hori T, Asoh S, Tuji A, Takemura T. Complete disappearance of unresectable hepatoblastoma by continuous infusion therapy through hepatic artery. J Pediatr Surg 1991;26:844–6.