Abstract
Background. Substantial survival may be observed with oligometastatic prostate cancer. Combining androgen deprivation (AD) and high-dose external beam radiotherapy (RT) to isolated regional or distant lesions may be proposed for these patients and the outcome of this strategy is the purpose of the present report. Material and methods. From 2003 to 2010, 50 prostate cancer patients were diagnosed with synchronous (n = 7) or metachronous (n = 43) oligometastases (OM). Among the relapsing patients, the recurrence occurred after radical prostatectomy in 33 patients and curative RT (± AD) in 10 patients. The median age at diagnosis was 63 years (range, 48–82). All patients underwent a bone scan and 18F-choline or 11C-acetate PET-CT at the time of diagnosis or relapse, showing regional and/or distant nodal and bone and/or visceral metastases in 33 and 17 patients, respectively. The median delivered effective dose was 64 Gy. All but one patient received neo-adjuvant and concomitant AD. Results. After a median follow-up of 31 months (range, 9–89) the three-year biochemical relapse-free survival (bRFS), clinical failure-free survival, and overall survival rates were 54.5%, 58.6% and 92%, respectively. No grade 3 toxicity was observed. Improved bRFS was found to be significantly associated with the number of OM. The three-year bRFS was 66.5% versus 36.4% for patients with 1 and > 1 OMs (p = 0.031). A normalised total dose (NTD in 2 Gy/fraction, alpha/beta = 2 Gy) above 64 Gy was also correlated with a better three-year bRFS compared to lower doses: 65% vs. 41.8%, respectively (p = 0.005). On multivariate analysis, only the NTD > 64 Gy retained statistical significance (HR: 0.37, 95% CI 0.15–0.93). Conclusion. Oligometastatic patients may be successfully treated with short AD and high-dose irradiation to the metastatic lesions. High dose improves bRFS. Such a treatment strategy may hypothetically succeed to prolong the failure-free interval between two consecutive AD courses.
Androgen deprivation (AD) therapy is indicated for patients with recurrent and/or metastatic prostate cancer [Citation1]. Long-term AD may induce substantial side effects in these patients negatively influencing general health status and quality of life such as cognitive and sexual impairment, fatigue, cardiovascular dysfunction, metabolic syndrome, loss of lean body mass, and osteoporosis [Citation2,Citation3]. Intermittent short courses of AD have been proposed as an alternative to continuous AD in order to reduce the severity and duration of the above mentioned side effects and to delay as much as possible the clinical appearance and progression of hormone-independent disease [Citation4,Citation5].
There is presently an increasing number of reports describing the existence of a proportion of prostate cancer patients who present with a reduced number of metastases (< 5 lesions) at relapse. This oligometastatic status has also been recognised in other tumour types such as melanoma, soft tissue sarcoma, liver, lung, and breast cancer, and has influenced the management of these malignancies in that a more radical treatment such as surgical resection has been employed [Citation6,Citation7]. The number of metastases may well reflect the biologic aggressiveness of the tumour and could determine the opportunity of potential curative interventions such as surgery or high-dose targeted radiotherapy (RT).
Positron emission tomography-computed tomography (PET-CT) studies with tracers such as choline or acetate are reliable tools to help with the diagnosis of oligometastatic disease after biochemical treatment failure in prostate cancer. An aggressive treatment combining AD and focussed high-dose irradiation for the isolated regional or distant lesions, as detected by PET-CT, may be proposed for these oligometastatic patients and is the purpose of the present report.
In this study we assessed the outcome of prostate cancer with 1–4 oligometastasis (OMs) treated sequentially or concomitantly with high-dose conformal RT and AD.
Material and methods
From March 2003 to November 2010, 50 patients were treated for synchronous (n = 7) or metachronous (n = 43) oligometastatic prostate cancer (i.e. 1 to 4 OM) at the University Hospital of Geneva, Switzerland (n = 28), and at the Teknon Oncologic Institute in Barcelona, Spain (n = 22). Among the 43 patients experiencing a recurrence, curative external beam RT (with or without AD) and radical prostatectomy (RP) were the two curative treatment modalities employed to treat the primary tumour in 10 and 33 patients, respectively. Fifty-one percent of patients failing surgery (n = 17) required postoperative salvage RT to the prostatic bed in addition to the planned treatment against their limited metastatic disease. Tumours and patients characteristics are shown in and . The median age at diagnosis was 63.2 years (range, 48–82). According to the National Comprehensive Cancer Network Guidelines, 39 (78%), six (12%) and three (6%) patients presented with high-, intermediate-, and low-risk disease at diagnosis, respectively. Risk was unknown in two patients.
Table I. Patients characteristics.
Table II. Disease characteristics: Number of treated metastases and anatomic sites.
The Phoenix definition, ‘nadir + 2 ng/ml’, for biochemical failure was used for the group of patients treated with external beam radiotherapy (EBRT). Failure after RP was defined as a postoperative PSA rise > 0.2 ng/ml. Among patients treated for recurrence, the interval between the diagnosis of prostate cancer and the biochemical relapse ranged from 1 to 114 months (median, 13 months). The median PSA value and the median PSA doubling time (PSA-DT) at the time of salvage were 6.7 ng/ml (range, 0.5–94) and 3.7 months (range 1–35), respectively. Thirty-five patients (70%) had a PSA-DT of ≤ 10 months.
All patients underwent a bone scan and a 18F-choline [Citation8] or 11C-acetate PET-CT [Citation9] which was performed at diagnosis in case of synchronic metastases or between 14 days and 5.8 years after the biochemical failure (median, 2.6 months). Six patients with contradictory results for bone metastases between the bone scan and the PET-CT underwent, in addition, a total body magnetic resonance imaging (MRI). Following these investigations, patients were diagnosed with regional (n = 21), distant nodal (n = 7), regional and distant nodal (n = 5), bone (n = 15), lung (n = 1), and bone and lung (n = 1) metastases. Twenty-eight patients (56%) presented with a single metastases only. In three patients initially treated with RP, the PET-CT also showed an abnormal tracer uptake in the prostatic bed compatible with a local relapse.
Neoadjuvant and concomitant AD using bicalutamide (50 mg qd, 30 days) and LH-RH analogue (goserelin) injections on a quarterly basis was given to all but one patient. The duration of AD was intended to be limited in time depending on age, comorbidities, tolerance, and PSA response (median 12 months, range 3–34 months). Most patients (n = 32) were treated for ≤ 12 months, and 11 patients were treated for ≥ 24 months. Hormonotherapy was not interrupted in two patients because of biochemical recurrence and multimetastatic progression of the disease while on treatment. In case of biochemical progression after a first salvage attempt AD was resumed according to the following criteria: a PSA threshold of 20 ng/ml for a PSA-DT ranging between 16 and 20 months; a PSA threshold of 10 ng/ml for a DT of 8–15 months; a PSA threshold of 4 ng/ml for a DT of 4–7 months; and any PSA value above 2 ng/ml for a DT ≤ 3 months.
All patients were treated with EBRT to the metastatic site/s and completed the treatment as planned. Radiotherapy was delivered using a three-dimensional (3D) conformal technique in 24 patients, IMRT in 17 patients, dynamic conformal arc therapy using one to four arcs in four patients, and volumetric modulated arc therapy in five patients. Patients treated with 3D conformal EBRT received an additional boost delivered with IMRT and dynamic arc therapy in six and eight patients, respectively.
All but one patient presenting with pelvic nodal relapse underwent an irradiation of the pelvic lymph node regions to a median dose 50.4 Gy, with a boost to the hypermetabolic lymph nodes seen on PET-CT to a maximum dose of 74.4 Gy (median 65 Gy, range 54–74). In the one remaining patient with a suspected local and a nodal relapse in the obturator region, the clinical target volume (CTV) included in the same boost volume the suspected obturator lymph node and the prostatic bed. Disease and treatment salvage characteristics are presented in . Fourteen patients with systemic metastases received a hypofractionated regimen using 28–36 Gy in 5–6 fractions. presents a PET-CT image of a patient with an isolated para-aortic nodal relapse and the corresponding treatment plan of the boost with arc-therapy.
Figure 1. (a) Single nodal paraaortic metastasis (arrow→) detected by 18F-Choline PET-CT from a high-risk prostate cancer patient. (b) Axial planning CT slices showing the dose distribution.
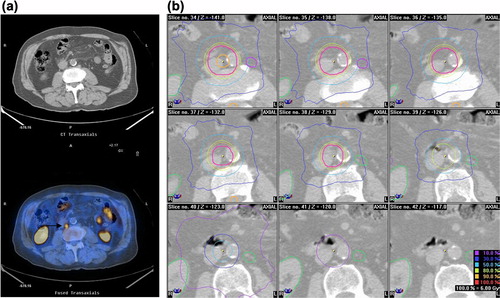
Table III. Treatment characteristics.
Patients were visited on status-check controls every week while on treatment and on follow-up visits six weeks after treatment completion and every six months thereafter. Treatment-related adverse events were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. A biochemical relapse was established at the time of a raising PSA value > 1 ng/ml, except for the seven patients presenting with synchronic metastases where the Phoenix definition was used. Biochemical relapse-free survival (bRFS), clinical failure-free survival (CFFS), and overall survival (OS) were assessed using the Kaplan-Meier method. CFFS was defined as the time from RT to development of new metastases. The log-rank test was used to compare different survival functions according to clinical [patient's age, primary tumour Gleason score, number or sites of metastases, PSA, and PSA-DT values at diagnosis or relapse and time of appearance of metastases (synchronous vs. metachronus)] and therapeutic factors (dose of irradiation). Multivariate analysis was performed by stepwise regression analysis to define the independent contribution of each factor. Differences were regarded as statistically significant at the p < 0.05 level. Analyses were performed on the Statistical Package for Social Sciences (SPSS) (Ver. 18.0, SPSS Inc., Chicago, IL, USA).
Results
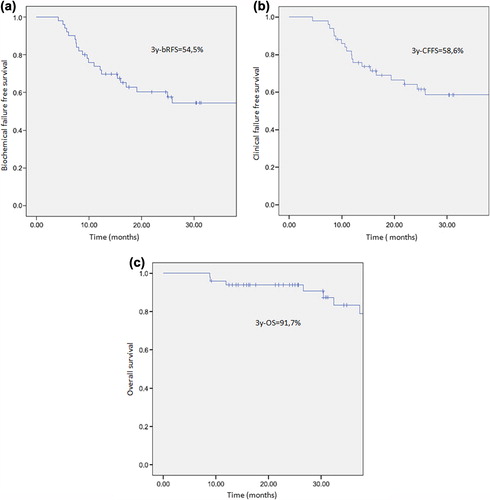
After a median follow-up of 31 months (range, 9–89) from RT completion, no patient developed > grade-2 acute or late toxicity. The three-year bRFS, CFFS, and OS were 54.5% (95% CI 39.6–69.4), 58.6% (95% CI 43.9–73.5), and 91.7% (95% CI 70.7–96.1), respectively (). All patients in remission at the most recent follow-up showed a normalised testosterone level with a mean± SD PSA value of 0.11 ± 0.2 ng/ml. Clinical disease progression with new metastases was observed in 25 patients (50%), after a median time of 14.4 months since the end of EBRT (range, 4–81). Among the relapsing patients, 21 developed multimetastatic disease and received AD with or without chemotherapy, with conventional local palliative RT if needed (one patient). The remaining five patients developed an oligometastatic recurrence in one or two sites (three patients and two patients, respectively). These patients received a short-term AD with high-dose RT as delivered previously. Seven patients died during the follow-up interval, all deaths related to prostate cancer progression.
Figure 2. (a) Biochemical relapse free survival. (b) Clinical failure free survival. (c) Overall survival.
On univariate analysis, the number of OM was a significant factor for bRFS. The estimated three-year bRFS was 66.5% versus 36.4% for patients treated to 1 and > 1 metastases, respectively (p = 0.031). A normalised total dose (NTD) in 2 Gy/fraction (alpha/beta = 2 Gy) above 64 Gy was also a significant prognostic factor for bRFS: the estimated three-year bRFS was 65% and 41.8% for patients receiving > 64 Gy and ≤ 64 Gy, respectively (p = 0.005). A statistical trend was observed, in addition, for bone versus non-bone metastases, the later presenting with a better three-year bRFS: 65.3% versus 32.4%, respectively (p = 0.077) and for a shorter PSA-DT (≤ 3.7 vs. > 3.7 months) at recurrence the longer DT associated with better bRFS (p = 0.067). Neither Gleason score, PSA value at salvage or at diagnosis of the primary disease for synchronic oligometastatic patients, the metastatic site (nodal vs. distant, bone vs. non-bone, nodal vs. non-nodal), nor the synchronous or metachronous status correlated significantly with bRFS. On multivariate analysis, only the NTD > 64 Gy retained statistical significance with an estimated HR of 0.37 (95% CI 0.15–0.93; p = 0.034).
Discussion
An oligometastatic status is not an uncommon clinical situation in patients with disseminated prostate cancer. Indeed, Harada et al. observed a large proportion of patients with < 3 metastases in an autopsy series on 136 patients: 36% and 32% of patients with one and two bone lesions, respectively [Citation10]. Only 10% of patients had ≥ 4 bone metastases. It has been suggested that the number of metastatic lesions in prostate cancer patients may be an important prognosticator. Singh et al. reported that patient with ≤ 5 metastases had a significantly better five-year OS compared to patients with > 5 metastases (73% vs. 45%) and similar to metastases-free patients [Citation11]. The authors concluded that oligometastatic patients may harbour tumours biologically less aggressive with limited metastatic potential and a slow growth rate of tumour cells. These patients could be suitable for more aggressive treatment. These results may be in keeping with our univariate analysis that showed that the number of OM (1 vs. > 1) was a favourable prognostic factor in bRFS.
Reports assessing the optimal management of patients with oligometastatic prostate cancer are rare. Indeed, a recent analysis by Milano et al. focused on stereotactic RT (SBRT) in 121 patients with ≤ 5 metastases from different primary origins except prostate cancer and reported a four-year disease control rate of 73% [Citation12]. Similarly, Bignardi et al. also reported on 19 non-prostate cancer patients treated with SBRT for lymph nodes metastases with a two-year rate of freedom from nodal progression of 78% [Citation13]. The good effect of hormones against metastatic prostate cancer may have been the reason to skip SBRT for those oligometastatic in these series.
Metastatic prostate cancer becomes most frequently resistant to continuous AD after an average treatment time of 2–3 years [Citation14]. Intermittent androgen suppression has been tested as a valid alternative to continuous AD in several Phase II–III trials [Citation15,Citation16]. These studies aimed to prolong the hormone-dependant status of the disease, as well as to reduce long-time lasting treatment-related side effects from AD while improving the overall quality of life during the off-treatment periods. In our study, the median duration of the first off-treatment period after the end of salvage RT was 19 months (0–82, range) at least comparable, if not better, than the first off-treatment period ranging from 6 to 16 months, as reported by others [Citation17].
There is no consensus on when to restart AD in an intermittent hormonotherapy setting when the PSA starts to progress after the first off-treatment period. This is why AD-free intervals reported by different authors are not comparable. In a prospective study, Yu et al. recruited 100 patients presenting with biochemical relapse after RT or RP [Citation18]. After nine months of AD, hormonotherapy was interrupted and restarted once the PSA value increased above 1 or 4 ng/mL in patients treated with RP or RT, respectively. The median duration of the first off-treatment interval was 9.5 months and was significantly correlated with PSA doubling time and with outcome. Bruchovsky et al. observed, however, that patients salvaged at a PSA value ≤ 10 ng/ml benefitted of a mean off-treatment period of 20 months [Citation19], similar to ours (19 months) and to the intermittent AD arm of the Canadian study by Crook et al. [Citation16] quoted above.
PSA-DT was the key factor to restart AD after successive biochemical failures in the present study. All but three patients progressed with a fast DT ≤ 4 months and benefitted only of a short off-treatment interval. These patients ultimately presented with multimetastatic disease. Five patients developed a second oligometastatic relapse and were eligible for a second salvage trial with high-dose targeted radiotherapy and AD. In our study, the relatively long median off-treatment interval above 19 months was mostly due to patients who were failure free after salvage AD and EBRT (mean PSA at last follow-up, 0.11 ng/ml).
A large majority of patients with biochemical failure after a curative treatment attempt of the primary tumour have no evidence of disease on standard imaging such as bone scan, abdominal CT or MRI at progression [Citation20,Citation21]. PET-CT imaging techniques with choline or acetate tracers are diagnostic procedures with a potentially higher predictive value than the standard imaging techniques quoted above. Scattoni et al. observed sensitivity and specificity rates for nodal metastases of 64% and 85%, respectively, with 11C-choline PET-CT imaging in patients with biochemical progression after prostatectomy [Citation22]. Delayed scanning with three phase 18F-choline PET-CT imaging may improve the positive-predictive value of such investigation by better discriminating benign lymph nodes from suspicious ones [Citation8]. In a recent study, Beheshti et al. assessed the predictive value of 18F-choline PET-CT in detecting metastatic bone disease in 70 prostate cancer patients. They observed a sensitivity and specificity rate of 79% and 97%, respectively [Citation23].
Despite the limited number of patients in our study (50 patients with a total of 79 metastases) this is, to our knowledge, the first report in the literature to have focused on the outcome of prostate cancer patients with equal or less than four metastases treated with a curative intend with high-dose EBRT in addition to limited AD. Jereczek-Fossa et al. have published recently a series with 19 patients presenting with single nodal (n = 16) or distant metastases (n = 3) at recurrence and salvaged with AD and SBRT (CyberKnife) [Citation24]. Roughly, 63% among those treated for pelvic nodal metastases, but none of those treated for distant metastases, were free of biochemical progression (i.e. two increases in PSA level relative to the pre-salvage value) at 30 months after treatment. Despite the more strict definition of biochemical failure in our series, Jereczek-Fossa et al. reported a 56% and 50% three-year bRFS rate among patients treated for pelvic nodal and for bone metastases, respectively.
Berardi et al. presented at ESTRO 2011 a study on 40 prostate cancer patients with pelvic/para-aortic (n = 36) or mediastinal (n = 4) nodal recurrences after RP [Citation25]. Those patients were treated with 11C-choline PET-CT guided moderate hypofractionated RT to high doses ranging from 42 Gy in 6 fractions to 74.2 Gy in 28 fractions with a simultaneous integrated boost. After a median follow-up of 16 months, a significant PSA reduction was documented in 37 of 40 patients. Among 19 patients with post-treatment PET-CT evaluation, a complete metabolic response was observed in 13 [Citation25]. Although the effect of irradiating OMs may have been overcome by AD treatment in our study artefactually biasing the favourable results obtained for these patients, a dose-response effect was observed with better bPFS figures when delivering > 64 Gy NED. This favourable correlation suggests, anyhow, a potential role of high-dose RT in addition to hormones for OMs patients.
Our data support the good tolerance level of our treatment proposal. In the CyberKnife trial quoted above, all treatments for bone metastatic lesions were free of acute toxicity, as were 15 of 16 pelvic lymph node irradiations [Citation24]. Bignardi et al. reported severe late effects after irradiating abdominal lymph nodes metastases in only one of 19 patients: grade 3 toxicity with sub-occlusive events [Citation13]. In the recent report by Berardi et al. no grade ≥ 2 late toxicity was reported.
Limitations of the current study include the small number of patients and the lack of a control group undergoing AD with only palliative RT to main metastases foci if needed. To our knowledge, only one randomised phase II trial comparing eradication of oligometastatic disease (up to three synchronous lesions) with stereotactic body RT or surgery versus active surveillance with the start of AD at time of progression has recently started recruiting patients (http://clinicaltrials.gov: NCT01558427). The primary end point is AD therapy-free survival. Fifty-four patients will be enrolled and estimated completion date is May 2017. The effect of the NTD on bRFS in our study is interesting. It is unlikely that this finding is related to a selection phenomenon as the dose differences are mainly due to radiation protocols evolving in our department thanks to better irradiation techniques available (arctherapy, IMRT). However, this finding has to be taken with caution and warrants further investigation.
In summary, prostate cancer patients presenting with ≤ 4 metastases at diagnosis or after failure following curative treatment of the local tumour may be successfully treated with limited AD and high-dose focused irradiation to the metastatic lesions. Such a treatment strategy may hypothetically succeed to prolong the failure free interval between two consecutive AD courses when patients are on intermittent AD salvage and deserves to be further tested in controlled randomised trials. The administered dose for the OM(s) was an independent favourable prognostic factor for bRFS.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 2007;12: 1596–605.
- Sadetsky N, Lubeck DP, Pasta DJ, Latini DM, DuChane J, Carroll PR. Insurance and quality of life in men with prostate cancer: Data from the Cancer of the Prostate Strategic Urological Research Endeavor. BJU Int 2008;6:691–7.
- Kyrdalen AE, Dahl AA, Hernes E, Hem E, Fossa SD. Fatigue in prostate cancer survivors treated with definitive radiotherapy and LHRH analogs. Prostate 2010;13:1480–9.
- Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer 1993;9:2782–90.
- Keizman D, Huang P, Antonarakis ES, Sinibaldi V, Carducci MA, Denmeade S, et al. The change of PSA doubling time and its association with disease progression in patients with biochemically relapsed prostate cancer treated with intermittent androgen deprivation. Prostate 2011;71:1608–15.
- Strong VE, D’Angelica M, Tang L, Prete F, Gonen M, Coit D, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 2007;12:3392–400.
- Fong Y, Brennan MF, Cohen AM, Heffernan N, Freiman A, Blumgart LH. Liver resection in the elderly. Br J Surg 1997;10:1386–90.
- Steiner C, Vees H, Zaidi H, Wissmeyer M, Berrebi O, Kossovsky MP, et al. Three-phase 18F-fluorocholine PET/CT in the evaluation of prostate cancer recurrence. Nuklearmedizin 2009;1:1–9; quiz N2–3.
- Albrecht S, Buchegger F, Soloviev D, Zaidi H, Vees H, Khan HG, et al. (11) C-acetate PET in the early evaluation of prostate cancer recurrence. Eur J Nucl Med Mol Imaging 2007;2:185–96.
- Harada M, Iida M, Yamaguchi M, Shida K. Analysis of bone metastasis of prostatic adenocarcinoma in 137 autopsy cases. Adv Exp Med Biol 1992;324:173–82.
- Singh D, Yi WS, Brasacchio RA, Muhs AG, Smudzin T, Williams JP, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases?. Int J Radiat Oncol Biol Phys 2004;1:3–10.
- Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008;3:650–8.
- Bignardi M, Navarria P, Mancosu P, Cozzi L, Fogliata A, Tozzi A, et al. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys 2011;81:831–8.
- Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998;15:1036–42.
- Malone S, Perry G, Eapen L, Segal R, Gallant V, Dahrouge S, et al. Mature results of the Ottawa phase II study of intermittent androgen-suppression therapy in prostate cancer: Clinical predictors of outcome. Int J Radiat Oncol Biol Phys 2007;3:699–706.
- Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy.NEngl J Med 2012;367:895–903.
- Bhandari MS, Crook J, Hussain M. Should intermittent androgen deprivation be used in routine clinical practice?. J Clin Oncol 2005;32:8212–8.
- Yu EY, Gulati R, Telesca D, Jiang P, Tam S, Russell KJ, et al. Duration of first off-treatment interval is prognostic for time to castration resistance and death in men with biochemical relapse of prostate cancer treated on a prospective trial of intermittent androgen deprivation. J Clin Oncol 2010;16: 2668–73.
- Bruchovsky N, Klotz L, Crook J, Goldenberg SL. Locally advanced prostate cancer – biochemical results from a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen recurrence after radiotherapy. Cancer 2007;5:858–67.
- Jacobson AF, Fogelman I. Bone scanning in clinical oncology: Does it have a future?. Eur J Nucl Med 1998;9:1219–23.
- Nakanishi K, Kobayashi M, Takahashi S, Nakata S, Kyakuno M, Nakaguchi K, et al. Whole body MRI for detecting metastatic bone tumor: Comparison with bone scintigrams. Magn Reson Med Sci 2005;1:11–17.
- Scattoni V, Picchio M, Suardi N, Messa C, Freschi M, Roscigno M, et al. Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: Results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur Urol 2007;2:423–9.
- Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Hammer J, et al. The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: Correlation with morphological changes on CT. Mol Imaging Biol 2010;1:98–107.
- Jereczek-Fossa BA, Beltramo G, Fariselli L, Fodor C, Santoro L, Vavassori A, et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys 2012;2:889–97.
- Berardi G, Pappalardi B, Fiorino C, Cozzarini C, Guazzoni G, Calandrino R. Hypofractionated tomotherapy treatment (HTT) in prostate cancer lymph nodal relapse detected by 11CCholine PET/CT. Radiother Oncol 2011;99(Suppl 1):S202.