To the Editor,
Cytotoxic treatment has been successfully established both in the adjuvant [Citation1] and in the palliative treatment [Citation2] of patients with pancreatic adenocarcinoma (PC). 5-fluourouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) is clearly the most effective regimen tested in phase III clinical trials for advanced PC patients with good performance status [Citation2]. Based on the high objective response rates observed with the FOLFIRINOX regimen, neoadjuvant treatment seems attractive when down-staging or down-sizing of the tumour is needed to achieve R0 resection in borderline resectable PC. Up to now, there has been limited evidence concerning the use of FOLFIRINOX as a neoadjuvant treatment strategy. To the best of our knowledge, there has been just one study published so far which addresses the role of neoadjuvant FOLFIRINOX for predominantly locally advanced PC patients [Citation3]. In this study, we report on the efficacy and toxicity of neoadjuvant FOLFIRINOX, and on the surgical results of this treatment modality. Between December 2010 and July 2012, all consecutive patients with good European Cooperative Oncology Group (ECOG) score, adequate liver function and borderline resectability were treated with FOLFIRINOX as a neoadjuvant treatment regimen. Resectability in terms of imaging was defined according to the consensus criteria of the American Hepato-Pancreato-Biliary Association (Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.771821) [Citation4]. Written informed consent was obtained from all patients. Pegfilgrastim was administered as primary prophylaxis in each cycle for all patients. Performance status had to be European cooperative oncology group (ECOG) 0 or 1 and bilirubin levels had to be < 1.5 × upper limit of normal prior to start of neoadjuvant treatment. Adverse events were defined according to Common Toxicity Criteria CTC 3.0. Response was evaluated after four cycles of treatment. Four to six cycles of FOLFIRINOX were administered and then, after interdisciplinary radiological assessment, the tumour was resected if no progression had been occurred. Tumour down-staging was assessed by comparing clinical stage (cT and cN) prior to treatment with pathological stage of the resected tumour. We also determined responses according to RECIST 1.1 criteria [Citation5]. All resections were performed by one experienced surgeon (HR, general and vascular surgeon). Follow- up was performed by clinical examination and measurement of tumour markers CA19-9 and CEA every three months and computed tomography (CT) scans every 3–6 months. Progression free survival (PFS) was defined as date of start of the first cycle of FOLFIRINOX until date of diagnosis of progression or recurrence or censoring by the date of last patient contact without progression or death. Overall survival (OS) was defined as date of start of FOLFIRINOX until death or censoring by the date of the last patient contact.
Results
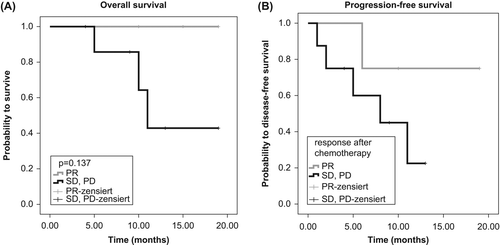
Overall, 10 patients with borderline resectable pancreatic adenocarcinoma and two patients with minimal metastases in the liver were included in our study. Seven of the 12 patients had to be treated with a biliary stent prior to chemotherapy due to cholestasis (five plastic stents and two metal stents). Toxicity of FOLFIRINOX was generally mild, grade 3 toxicity was seen in 4/12 patients and 4/53 cycles. Grade 4 toxicity (pulmonary embolism) was observed in one patient. Hospitalisation was necessary in one patient because of grade 3 diarrhoea. Toxicity was similar in the seven patients with a biliary stent compared to the patients not receiving a stent before FOLFIRINOX. According to RECIST criteria there was a partial remission in four patients, stable disease in six patients and progressive disease in two patients. Down-staging could be accomplished in five of 12 patients. Surgery could be performed in 10 of 12 patients. A Caush Whipple pancreatoduodenectomy procedure or a pylorus preserving pancreatoduodenectomy were performed in eight cases, a total pancreatectomy in one case and a left pancreatic resection in one case. Vascular resection was performed in four patients (three patients with portal vein resection, one patient with superior mesenteric vein resection and three patients with right hepatic artery resection), resection of a solitary liver metastasis was performed in two patients. Surgical treatment-related mortality at day 30 was 0/10, at day 60 1/10 (due to liver abscess and sepsis). Median follow-up after start of chemotherapy was 15.4 months. The two patients with progression and irresectability have died 10.3 and 11.0 months, respectively, after the start of treatment. Regarding OS and PFS, the Kaplan-Meier curve analyses reveal numerically superiority for patients (n = 4) with an objective response (PR) followed by resection compared to patients (n = 8) with stable disease or progressive disease with or without resection (). Survival data are not mature yet, but nine patients are still alive after a median 15.4 months of observation and seven of these are still in complete remission.
Figure 1. (A) Kaplan-Meier curve for overall survival of patients with partial remission compared to patients without remission (p = 0.137, log-rank test). (B) Kaplan-Meier curve for progression-free survival of patients with partial remission compared to patients without remission (p = 0.237, log-rank test).
Discussion
During the last years, borderline resectable pancreatic adenocarcinoma has been defined as a new subset of PC with a high rate of R1- or R2- resection if primary resection was performed [Citation6]. Prognosis of borderline resectable PC was shown to be worse compared to resectable tumours [Citation7]. This finding may also be caused by an increased rate of nodal involvement in patients with infiltration of the portal or superior mesenteric vein [Citation8], but also by the low sensitivity and specificity of preoperative imaging for prediction of resectability [Citation9]. However, patients with borderline resectable PC who achieved a R0 resection and those with down-staging after neoadjuvant treatment have the same prognosis as initially resectable cases [Citation10,Citation11]. Therefore, neoadjuvant treatment has been recommended by the NCCN 2012 guidelines and many institutions have administered preoperative chemotherapy and/or chemoradiation [Citation12–14]. The most effective palliative regimen in terms of response rates and survival in patients with PC, tested in phase III trials, is FOLFIRINOX [Citation2]. Therefore, we evaluated the feasibility and efficacy of FOLFIRINOX for neoadjuvant treatment in 12 patients with borderline resectable or minimally metastatic disease. To the best of our knowledge, only one report has used this regimen predominantly in locally advanced patients [Citation3]. In contrast to ours, the series by Hosein et al. included only four patients with borderline resectable PC [Citation3]. In our middle European series of 12 patients with a good performance status and primary administration of pegfilgastrim, FOLFIRINOX was well tolerated. In the ACCORD-11 study [Citation2], febrile neutropenia occurred in 5.4% of treated patients and no cholangitis was reported, although 15% of the patients had obtained a biliary stent. In line with these data, we also did not observe cholangitis in our seven patients with a biliary stent which suggests that prior stenting should not be an exclusion criterion for neoadjuvant FOLFIRINOX treatment. Beside the safety, we saw very rapid responses to this treatment and, therefore, usually administered only four treatment cycles. Although the optimal treatment duration has to be established in larger prospective trials, an extended treatment might have resulted in higher response rates but also in increased toxicity, higher peri-operative morbidities and mortalities as well as higher risk of progression resulting in unresectability and the risk of deterioration of performance status. Survival data appear to be numerically superior for patients reaching an objective response to neoadjuvant treatment according to RECIST criteria, which may indicate that sensitivity to cytotoxic treatment is a prognostic factor and that these patients may benefit the most from resection. However, due to the low number of patients and the short period of follow-up, the differences for OS and for PFS did not reach statistical significance. Our findings should be confirmed in larger trials, which might underline that the remission status after chemotherapy may serve as a selection criterion for performing surgery in patients with borderline resectable PC. In conclusion, our data suggest that neoadjuvant FOLFIRINOX is safe when adequate performance status and liver function of patients are present. Further prospective trials are warranted to establish the exact role of this regimen as a neoadjuvant treatment modality and to define the predictive value of treatment response as a selection criterion for surgery in borderline resectable pancreatic adenocarcinoma.
Supplementary Table I.
Download PDF (17.3 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007;297:267–77.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med 2011;364:1817–25.
- Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199.
- Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol 2009;16:1727–33.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
- Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035–46.
- Wang J, Estrella JS, Peng L, Rashid A, Varadhachary GR, Wang H, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:3801–11.
- Siriwardana HP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg 2006; 93:662–73.
- Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: Criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. Am J Roentgenol 1997;168:1439–43.
- Stokes JB, Nolan NJ, Stelow EB, Walters DM, Weiss GR, de Lange EE, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619–27.
- Willett CG, Lewandrowski K, Warshaw AL, Efird J, Compton CC. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993;217:144–8.
- Bickenbach KA, Gonen M, Tang LH, O’Reilly E, Goodman K, Brennan MF, et al. Downstaging in pancreatic cancer: A matched analysis of patients resected following systemic treatment of initially locally unresectable disease. Ann Surg Oncol 2012;19:1663–9.
- Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg 2008;206:833–46; Discussion 46–8.
- Papalezova KT, Tyler DS, Blazer DG, 3rd, Clary BM, Czito BG, Hurwitz HI, et al. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer?J Surg Oncol 2012;106:111–8.