To the Editor,
Oesophageal cancer is associated with poor prognosis despite evolution in multimodality treatment (surgery, chemotherapy and radiotherapy). Definitive or pre-operative chemo-radiotherapy is used to treat locally advanced inoperable disease [Citation1,Citation2]. Local disease control specifically within the gross tumour volume (GTV) remains a problem with high local failure rates (45–58%) [Citation1,Citation3]. Increase in radiation dose is an independent predictor of pathological complete response; dose escalation to tumours at other sites has demonstrated improved local control and survival [Citation3,Citation4]. To facilitate this, improvement in radiotherapy delivery is necessary. VMAT has been shown to be dosimetrically favourable when compared to both three-dimensional conformal (3DCRT) and intensity-modulated radiotherapy (IMRT) techniques for radical treatment of distal oesophageal and gastro-oesophageal (GOJ) tumours [Citation5].
Conventionally large volumetric expansions are applied to the tumour to create the target radiotherapy volume given: 1) the high risk of submucosal microscopic spread along the oesophagus; these expansion margins have been validated by clinical and pathological studies [Citation6,Citation7]; and 2) to account for both inter-and intra-fractional target motion and set up variations in order to minimise geographical misses [Citation8–11].
Tumour shape and position varies during radiotherapy as a result of normal oesophageal peristalsis, adjacent cardiac motion and breathing [Citation9,Citation12]. Studies in lung cancer, demonstrate the normal distal oesophagus has greatest mobility [Citation13,Citation14]. Therefore in order to assure target coverage for 95% of fractions a 9–16 mm margin in the medial to lateral direction and a 8–12 mm margin in the anterior to posterior direction is suggested for treatment of oesophageal cancer [Citation11,Citation13,Citation14]. GOJ tumours also demonstrate significant degree of motion associated with breathing. Two studies have used 4DCT to characterise this, both show that the magnitude of tumour motion was greatest below the diaphragm [Citation15,Citation16].
The radiotherapy planning computed tomography (CT) scan is normally acquired with the patient breathing freely. 4DCT scanning enables correlation of CT data acquisition to the respiratory cycle. Alternatively, actual tumour motion during radiotherapy treatment delivery caused by breathing can be managed using Active Breathing Control (ABC) (Elekta Ltd, Crawley, UK). This technique delivers dose at a predetermined point in the breathing cycle. The aim of this study is to determine whether ABC gating and 4DCT improves motion management to individualise patient margins and reduce dose to OARs.
Material and methods
Patients with pathologically confirmed lower third oesophageal or GOJ tumours suitable for radical radiotherapy were recruited from the Royal Marsden Hospital, Sutton to an institutional research committee approved protocol.
Nine patients consented to the study between June 2008 and November 2010. The patient and tumour characteristics are detailed in Supplementary Table I (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.776174).
For radiotherapy treatment planning purposes an initial contrast-enhanced free-breathing helical planning CT scan (CECT) was acquired with a slice thickness of 2.5 mm on a large bore CT scanner (Philips Medical Systems, Cleveland, OH, USA). 4DCT image acquisition occurred immediately after this.
The 4DCT data set was rigidly fused with the standard free-breathing CECT. The exhale data set was chosen to represent breath hold (BH) and was considered equivalent to an ABC BH scan.
The GTV was defined as the volume of the primary tumour seen on CT with the aid of all available diagnostic staging information. The GTV was contoured on the axial images of the CECT and on the exhale and inhale 4DCT reconstruction using the Pinnacle planning software (Philips Medical Systems, Madison, WI, USA) v9.0h. To eliminate inter-observer variation the same physician performed all outlining.
To account for microscopic spread in all cases the CTV was created from the GTV by contouring along the axis of the oesophagus for 3 cm cranial-caudally for squamous cell carcinomas, 3 cm cranially and 5 cm caudally for adenocarcinomas; with a 0.5 cm circumferential margin. To create the standard PTV, a 1 cm margin was applied in the superior-inferior direction with 0.5 cm circumferentially to account for motion, with a uniform 0.5 cm expansion to account for set up error.
Using the 4DCT data set, two additional PTVs were generated. A composite volume using GTV inhale and GTV exhale was created. This was edited using all of the 10 respiratory phases to create an ITV. This was expanded as above to create the CTV. A further 0.5 cm isotropic margin was added to account for set up error in order to create the 4D PTV.
The GTV at exhale was expanded as above to create the CTV, with an additional 0.5 cm isotropic margin to account for set up error to create the breath hold PTV (BH PTV).
Organs at risk (heart, both lungs and spinal cord) were contoured on the free breathing CT.
To quantify target volume motion the centre of mass (COM) coordinates were calculated using Pinnacle for all GTVs at maximal inhalation and exhalation.
Patients were planned using in house software AutoBeam v5.1 [Citation17]. The objectives and dose constraints used for the VMAT inverse planning are outlined in . The dose prescribed was 54 Gy in 30 daily fractions (1.8 Gy per fraction).
Table I. Objectives and constraints for oesophageal VMAT planning.
Three VMAT plans were created for each patient using the standard PTV, 4D PTV and BH PTV (Supplementary Figure 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.776174).
Each plan was evaluated in accordance with ICRU recommendations [Citation18–20] using dose-volume histograms (DVHs).
To assess the uniformity of the absorbed dose distribution within the target volume dose homogeneity index (HI) was calculated as follows:
A value of HI of 0 indicated a homogeneous dose distribution [Citation20].
To assess conformity of the high dose region to the target volume the van't Reit conformation number (CN) was used [Citation21]:
where TVri represented the target volume (TV) covered by the reference isodose (95% in this case); TV was the PTV volume being assessed and Vri was the total volume encompassed by the reference isodose (95%). A value close to 1 represented high conformity [Citation20].
Dose to critical normal tissue, specifically volume of lung irradiated to 5 Gy and 20 Gy (V5 and V20); mean cardiac dose and heart volume irradiated to 30 Gy and 40 Gy (V30 and V40); maximum spinal cord dose and volume of liver irradiated to 30 Gy (V30) were calculated for each plan from DVH data.
Data were analysed using SPSS statistical software v17 (IBM software). Wilcoxon signed ranks test (with a significance level of 0.05) was used to compare the measured change in tumour volumes and to evaluate change in normal tissue irradiation between the three plans.
Results
The mean GTV volumes on the standard free breathing CECT scan (GTV standard) was 59.3 cm3 (range 27.8–109.8 cm3). The mean GTV on maximal inhalation was 57.3 cm3 (range 22.8–94.8 cm3) and mean GTV on maximal exhalation was 56.7 cm3 (range 26.9–97.9 cm3). No significant difference in volume between CECT GTV and inhale and exhale 4DCT GTV was seen.
The mean GTV centroid displacement in each direction is presented in Supplementary Table II (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.776174). Greatest overall motion was seen in the superior to inferior direction (mean 1.02 cm, range 0.29–1.54 cm).
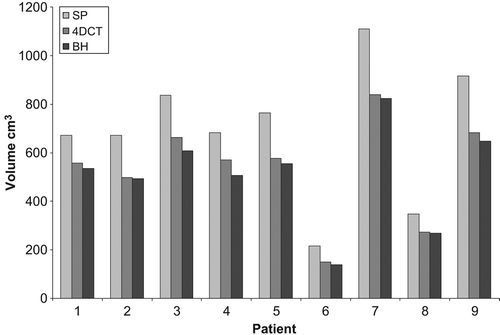
The population mean PTV for the standard plan, 4D plan and BH plan were 690 cm3 (range 215–1109 cm3), 534 cm3 (range 150–839 cm3) and 508 cm3 (range 139–823 cm3), respectively. The reduction in PTV was statistically significant between the standard PTV and 4D PTV (p = 0.008), standard PTV and BH PTV (p = 0.008) and 4D and BH PTV (p = 0.008). 4D and BH PTVs were reduced by a mean (± SD) of 22.6% (± 4.5%) and 26.4% (± 4.3%), respectively, when compared with the standard PTV. represents the comparative individual volume reduction in PTV.
Plan evaluation and target coverage
A summary of the PTV statistics is presented in . There was no significant difference in target coverage characteristics including HI and CN between the three plans. There was a reduction in the mean (range) PTV 95% coverage from 95% (85–100%) for the standard plan, 91% (84–100%) for the 4D plan and 91% (84–98%) for the BH plan. This was because following optimisation of the standard plan the same parameters were applied to the 4D plan and BH plan to ensure any changes seen were as a result of the new margins and not because of alteration in the prescription point etc. All plans were clinically acceptable and implementable.
Table II. Summary of target coverage.
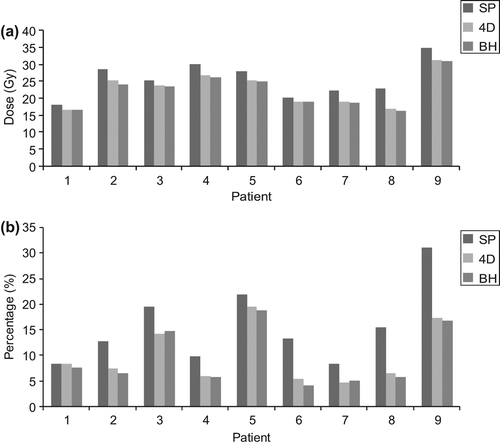
All plans satisfied the specified dose constraints. There was a reduction in mean lung V20 from 15.6% (range 8.3–30.9%) to 10.0% (range 4.8–17.3%) (p = 0.02) in the 4D plan and to 9.5% (range 4.2–18.7%) (p = 0.01) in the BH plan when compared to the standard plan (). There was 12% reduction in mean lung V5 when 4D plan was compared to standard (p = 0.28). The BH plan reduced mean V5 by 2.7% when compared to standard plan (p = 0.52), but was 11% greater when compared to 4D plan (p = 0.31).
For the 4D and BH plans there was a reduction in the volume of heart irradiated to higher doses (> 30 Gy), known to correlate with cardiac toxicity [Citation22]. At V30, there was 21% reduction (p = 0.01) in the irradiated volume using the 4D plan and 26% reduction (p = 0.01) using the BH plan when compared to the standard plan. At V40, both the 4D plan and the BH plan provided further reduction of 28% (p = 0.007) and 30% (p = 0.007), respectively, when compared to standard plan.
Significant improvement in liver V30 and spinal cord maximum point dose (up to 26%) with 4D and BH plans were also seen. A summary of the normal tissue DVH is presented in Supplementary Table III and an example DVH is shown in Supplementary Figure 2 (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.776174).
Discussion
The ICRU 62 recommends explicit inclusion of tumour motion in the radiotherapy treatment planning process. If greater than 5 mm motion is observed in any direction or if significant normal tissue sparing can be achieved, respiratory management techniques are recommended [Citation19,Citation23]. Given the established motion of the distal oesophagus and GOJ with breathing we advocate 4DCT guided or gating techniques to be incorporated into radiotherapy treatment delivery to tumours in this region [Citation13–16].
Population-based expansions in most instances overestimate tumour motion which leads to unnecessary normal tissue irradiation. In this study individualised expansions based on actual tumour motion demonstrate statistically significant reduction in dose delivered to all pre-defined OARS when the standard plan is compared to the 4D plan. When the BH plan is compared to standard plan there is significant reduction in lung V20, mean heart dose, heart V30 and V40, liver V30 and maximum cord dose. When the BH plan was compared to the 4D plan a further significant reduction was seen in mean heart dose (2% p = 0.024), liver V30 (11% p = 0.012) and maximum cord dose (4% p = 0.021).
Breath hold was performed in maximum exhalation for this study including GOJ and distal tumours because the liver position was more reliably reproduced. This did not adversely affect dose to the liver as target constraints were comfortably met for both 4D and BH plans (mean liver V30 for 4D plan was 6.8% (range 0–15) and BH plan 6.1% (range 0–14)). In a dosimetric study of respiratory gated radiotherapy for oesophageal tumours, breath hold in inspiration has been found to reduce cardiac V40 and lung V20 (24). Adequate volume of unexposed lung sparing is also important. Volumes of lung irradiated to 5 Gy and greater are associated with significant postoperative pulmonary morbidity, including pneumonia and acute respiratory distress syndrome in those who receive neoadjuvant chemoradiotherapy prior to definitive surgery [Citation25]. Radiotherapy techniques such as VMAT create a low dose bath outside the PTV as compared to 3DCRT. For the study population, the 4D plan improved the lung V5 as compared to the standard plan but the BH plan created in exhalation had worse lung V5 than the 4D plan.
Patients with oesophageal cancer commonly have co existent chronic obstructive airways disease (COPD) and other significant co-morbidity. All these factors may impact on confidently reproducing initial breathing patterns [Citation26]. They may also make clinical implementation of gating techniques unacceptable and intolerable for the patient. Other physiological activity including peristalsis, organ filling and adjacent organ movement (cardiac and stomach) contributes to oesophageal motion. In this study tumour motion was comparable to previous work. Lateral tumour motion was greatest to the right, probably reflecting impact of liver movement. Although 4DCT generates a composite volume of tumour position over time, margins based on gated techniques alone do not control for this.
Normal tissue sparing demonstrated with respiratory adaptive techniques will be increasingly important with the integration of new concurrent chemotherapy regimes which are known to reduce normal tissue tolerance [Citation27]. Biological agents such as epidermal growth factor receptor (EGFR) inhibitors, anti Her-2 antibody and anti vascular endothelial growth factor receptor (VEGFR) antibody are all under investigation for the treatment of upper GI malignancies. Therefore additional dose sparing gains to OARs may be important in future treatment development with novel radio-sensitising agents with overlapping toxicities.
High risk of local regional relapse after chemoradiotherapy justifies investigation of dose escalation within clinical trials using modern radiotherapy techniques. Combining these techniques with respiratory gating could facilitate the reduction of dose received by normal tissue and allow safe delivery of higher doses.
Respiratory adapted radiotherapy delivery appears to be clinically justifiable and has been demonstrated to be an important consideration in other tumour types such as lung and liver radiotherapy. Further work however needs to be done to verify if the motion as measured by the planning 4DCT has the same characteristics and reflects motion occurring during radiotherapy delivery.
Supplementary Figures 1–2
Download PDF (834.5 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
We acknowledge NHS funding to the NIHR Biomedical Research Centre.
References
- Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–74.
- Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. [Clinical Trial Comparative Study Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.] 1992;326:1593–8.
- Geh JI, Bond SJ, Bentzen SM, Glynne-Jones R. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: Evidence of a radiation and chemotherapy dose response. Radiother Oncol [Meta-Analysis Review] 2006;78:236–44.
- Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/ unresectable non-small-cell lung cancer: Long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324–33.
- Hawkins MA, Bedford JL, Warrington AP, Tait DM. Volumetric modulated arc therapy planning for distal oesophageal malignancies. Br J Radiol 2012;85:44–52.
- Hosch SB, Stoecklein NH, Pichlmeier U, Rehders A, Scheunemann P, Niendorf A, et al. Esophageal cancer: The mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol [Research Support, Non-U.S. Gov’t] 2001;19:1970–5.
- Gao XS, Qiao XY, Wu FP, Cao L, Meng XL, Dong ZM, et al. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys 2007;67:389–96.
- Lorchel F, Dumas JL, Noel A, Wolf D, Bosset JF, Aletti P. Esophageal cancer: Determination of internal target volume for conformal radiotherapy. Radiother Oncol [Research Support, Non-U.S. Gov’t] 2006;80:327–32.
- Cohen RJ, Paskalev K, Litwin S, Price RA, Feigenberg SJ, Konski AA. Esophageal motion during radiotherapy: Quantification and margin implications. Dis Esophagus 2010;23: 473–9.
- Yamashita H, Haga A, Hayakawa Y, Okuma K, Yoda K, Okano Y, et al. Patient setup error and day-to-day esophageal motion error analyzed by cone-beam computed tomography in radiation therapy. Acta Oncol 2010;49:485–90.
- Patel AA, Wolfgang JA, Niemierko A, Hong TS, Yock T, Choi NC. Implications of respiratory motion as measured by four dimensional computed tomography for radiation treatment planning of esophageal cancerInt J Radiat Oncol Biol Phys 2009;74:290–6.
- Hashimoto T, Shirato H, Kato M, Yamazaki K, Kurauchi N, Morikawa T, et al. Real-time monitoring of a digestive tract marker to reduce adverse effects of moving organs at risk (OAR) in radiotherapy for thoracic and abdominal tumors. Int J Radiat Oncol Biol Phys 2005;61:1559–64.
- Michalski D, de Andrade RS, Heron DE, Huq MS. Four-dimensional computed tomography (4D-CT)-based analysis of intra-fractionall esophageal motion. Int J Radiat Oncol Biol Phys 2007;69:S518–9.
- Dieleman EMT, Senan S, Vincent A, Lagerwaard FJ, Slotman BJ, de Koste JRV. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration. Int J Radiat Oncol Biol Phys 2007;67:775–80.
- Zhao KT, Liao ZX, Bucci MK, Komaki R, Cox JD, Yu ZQH, et al. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageat junction. Radiother Oncol 2007;84:283–9.
- Yaremko BP, Guerrero TM, McAleer MF, Bucci MK, Noyola-Martinez J, Nguyen LT, et al. Determination of respiratory motion for distal esophagus cancer using four-dimensional computed tomography. Int J Radiat Oncol Biol Phys 2008;70:145–53.
- Bedford JL. Treatment planning for volumetric modulated arc therapy. Medical physics 2009;36:5128–38.
- 50IR. Prescribing, recording and reporting photon beam therapy. International Commission on Radiation Units and Measurements (ICRU). 1993.
- 62IR. Prescribing, recording and reporting photon beam therapy. International Commission on Radiation Units and Measurements (ICRU). 1999.
- 83IR. Prescribing, recording and reporting photon beam intensity-modulated radiation therapy. International Commission on Radiation Units and Measurements (ICRU). Journal of the ICRU. 2010.
- van’t Riet A, Mak AC, Moerland MA, Elders LH, van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: Application to the prostate. Int J Radiat Oncol Biol Phys 1997;37:731–6.
- Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys [Meta- Analysis Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] 2010;76(3 Suppl): S77–85.
- Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006;33:3874–900.
- Turner LM, Howard JA, Dehghanpour P, Barrett RD, Rebueno N, Palmer M, et al. Exploring the feasibility of dose escalation positron emission tomography-positive disease with intensity-modulated radiation therapy and the effects on normal tissue structures for thoracic malignancies. Med Dosim 2011;36:383–8.
- Wang SL, Liao Z, Vaporciyan AA, Tucker SL, Liu H, Wei X, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys [Research Support, Non-U.S. Gov’t] 2006;64: 692–9.
- Korreman SS, Juhler-Nottrup T, Boyer AL. Respiratory gated beam delivery cannot facilitate margin reduction, unless combined with respiratory correlated image guidance. Radiother Oncol 2008;86:61–8.
- O’Connor BM, Chadha MK, Pande A, Lombardo JC, Nwogu CE, Nava HR, et al. Concurrent oxaliplatin, 5-fluorouracil, and radiotherapy in the treatment of locally advanced esophageal carcinoma. Cancer J 2007;13: 119–24.