Abstract
Background. To evaluate the safety and efficacy of moderate-to-high intensity aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Methods. Twenty patients with stage IIB–IIIC operable breast cancer were randomly assigned to receive doxorubicin plus cyclophosphamide (AC) or AC in combination with aerobic training (AC + AET) (n = 10/group) for 12 weeks. The AC+ AET group performed three supervised aerobic cycle ergometry sessions per week at 60%–100% of exercise capacity (VO2peak). Safety outcomes included exercise testing as well as treatment- and exercise training-related adverse events (AEs), whereas efficacy outcomes included cardiopulmonary function and patient-reported outcomes (PROs) as measured by a cardiopulmonary exercise test (CPET) and Functional Assessment of Cancer Therapy-Breast (FACT-B) scale. Results. Twelve non-significant ECG abnormalities and three non-life threatening events occurred during CPET procedures. One AE was reported during aerobic training. There were no significant between group differences for clinician-documented events (e.g. pain, nausea) or hematological parameters (p's > 0.05). Attendance and adherence rates to aerobic training were 82% and 66%, respectively. Intention-to-treat analysis indicated that VO2peak increased by 2.6 ± 3.5 ml/kg/min (+ 13.3%) in the AC + AET group and decreased by 1.5 ± 2.2 ml/kg/min (−8.6%) in the AC group (between group difference, p = 0.001). FACT-B increased 11.1 points in the AC + AET group compared to a 1.5 point decrease in the AC group (between group difference, p = 0.685). Conclusion. Moderate-to-high intensity aerobic training when conducted with one-on-one supervision is a safe adjunct therapy associated with improvements in cardiopulmonary function and select PROs during neoadjuvant chemotherapy.
The past decade has witnessed increased clinical and research interest in the application of exercise training (herein referred to as aerobic training) following a cancer diagnosis [Citation1,Citation2]. Recent systematic reviews and meta-analyses conclude that aerobic training is a safe and effective method to improve cardiopulmonary function and patient-reported outcomes (PROs) such as quality of life (QOL), fatigue, and depression both during and following the completion of primary adjuvant therapy in patients with solid malignancies [Citation1,Citation2]. Based on the extant literature, several national and international agencies have published cancer-specific general exercise guidelines recommending exercise participation for all persons following a cancer diagnosis [Citation1,Citation3,Citation4]. Despite significant progress, questions remain regarding the safety and efficacy of aerobic training in the oncology setting, particularly during adjuvant therapy.
The current evidence suggests that aerobic training is safe during primary adjuvant therapy. However, only 14% of studies reported monitoring adverse events (AEs) during the study conduct [Citation5]. Thus, it is not clear whether the low incidence of AEs reflects the true safety of aerobic training or less than optimal monitoring and/or reporting. Relatedly, the vast majority of studies to date in the oncology setting have evaluated the efficacy (and safety) of aerobic training programs following standard exercise prescription guidelines (i.e. 3–5 days per week at 50–75% of baseline exercise capacity for 12–15 weeks). Importantly, several recent randomized trials have demonstrated that exercise training prescriptions incorporating high-intensity aerobic training (i.e. 85–95% of baseline heart rate peak) elicits superior improvements in exercise capacity and other cardiovascular outcomes compared with standard moderate-intensity exercise prescriptions [Citation6–8].
Few studies have tested the efficacy of exercise prescriptions that incorporate high-intensity aerobic interval training in cancer patients especially those receiving chemotherapy [Citation9–11]. A potential explanation for this finding is that chemotherapy causes a broad range of adverse toxicities including bone marrow suppression leading to a high incidence of anemia and neutropenia, [Citation12] and aerobic training at high-intensities may have the potential to further compromise bone marrow [Citation13]. However, at present, there is no evidence to support this notion in the oncology setting. Nevertheless, prior to launching large-scale randomized trials investigating the efficacy of exercise prescriptions incorporating high- intensity training, it is prudent to evaluate the safety of such approaches in small, well-controlled studies utilizing standardized safety endpoints in conjunction with established measures of efficacy.
Accordingly, we conducted a pilot phase II randomized trial to investigate the safety of supervised moderate-to-high intensity aerobic training in women with operable breast cancer initiating anthracycline-containing neoadjuvant chemotherapy. Effects on cardiopulmonary function (e.g. exercise capacity) and PROs were also assessed. We hypothesized that aerobic training would be safe and confer significant improvements in cardiopulmonary function and PROs compared to usual care (neoadjuvant chemotherapy only).
Methods
Patients and procedures
Women with newly diagnosed, histologically confirmed unresected stage IIB–IIIC breast adenocarcinoma scheduled for first-line neoadjuvant chemotherapy at Duke Cancer Institute (DCI) were eligible for study participation. Other major eligibility criteria were: 1) Karnofsky performance status > 70; 2) no previous history of malignancy; 3) absence of significant cardiac disease; 4) absence of contraindications to neoadjuvant chemotherapy; 5) no absolute contraindications to supervised aerobic training based on a CPET; 6) willingness to travel to DCI to attend supervised aerobic training sessions three times a week; and 7) primary attending oncologist approval (determination of eligibility was at the discretion of the attending oncologist).
Following oncologist approval, eligible patients were provided with a thorough review of the study and asked if they were willing to participate. Interested participants completed an incremental cardiopulmonary exercise test (CPET), resting echocardiogram, and self-administered questionnaire to assess PROs (i.e. QOL, fatigue). Following successful completion of all baseline assessments, participants were randomly allocated to study groups as described below. All study procedures were reviewed and approved by the Duke University Medical Center (DUMC) institutional review board. All subjects signed a written consent prior to the initiation of any study-related procedures.
Randomization and blinding
Participants were randomly assigned to receive neoadjuvant doxorubicin and cyclophosphamide (AC alone) or neoadjuvant AC in combination with aerobic training (AC + AET) in a 1:1 ratio using a computer-generated program (n = 10/group). The allocation sequence was concealed from the study coordinator who assigned participants to groups. It was not possible to blind participants or exercise staff to group assignment, however, study exercise physiologists conducting the baseline and post- intervention (12 weeks) assessments were blinded to group assignment.
Therapy
Neoadjuvant chemotherapy consisted of four cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every three weeks (i.e. 12 weeks in duration). Prior to initiation of therapy, patients underwent clinical cardiac assessments including resting electrocardiograph (ECG) and echocardiogram. Participants randomized to AC alone were instructed to maintain their usual exercise levels throughout the duration of the study.
Aerobic training
Aerobic training consisted of three, one-on-one (non-group based) supervised cycle ergometry sessions per week on non-consecutive days for 12 weeks. In week 1, exercise intensity was initially set at 60% of baseline peak workload for 15–20 minutes duration. Duration and/or intensity were then subsequently increased throughout weeks 2–4 up to 30 minutes at 65% peak workload. In weeks 5 and 6, exercise intensity varied between 60% and 65% of peak workload for 30–45 minutes duration for two sessions; in the remaining session, participants cycled for 20–25 minutes at ventilatory threshold determined by a systematic increase in the VE/VO2 ratio while VE/VCO2 remained constant. From the seventh week onwards, participants performed two sessions at 60–70% peak workload with one threshold workout for 20–30 minutes. Finally, in weeks 10–12, participants performed two sessions at 60–70% peak workload with one interval session at 100% peak workload. Interval workouts consisted of 30 seconds at peak workload followed by 60 seconds of active recovery for 10–15 intervals. We have adopted this prescription approach in our prior work [Citation10,Citation11].
Study outcome assessments
The CPET, echocardiogram, and self-administered questionnaire were conducted at baseline and post-intervention (12 weeks) whereas treatment-related events were serially assessed across the study (i.e. baseline, 3, 6, 9, and 12 weeks). Exercise-related events were monitored during CPET procedures and aerobic training sessions.
Outcome assessments
Adverse events monitoring/safety. Treatment-related AEs were evaluated to assess the following clinician-documented AEs: nausea, myalgia, pain, alopecia, arthralgia, infection or presumed infection, fever or febrile neutropenia, and emergency room admittance. Serial changes in complete blood counts (CBCs) were also abstracted via medical chart review. Aerobic training-related AEs included resting and exercise vitals [e.g. heart rate, blood pressure, and arterial oxygen saturation (SpO2)] were monitored and recorded at each aerobic training session. All AEs during aerobic training were monitored and reported on the participant case report forms. In the present study, safety was operationalized as follows: assuming a true severe AE rate in the AC group of 4%, the study has 80% power with a one-tailed test conducted at the 0.05 level of significance to detect an increase to 47% in the AC + AET group.
Aerobic training attendance and prescription adherence. Attendance was calculated as the number of exercise sessions attended divided by the total number of planned sessions (i.e. 36 total planned sessions). Adherence to the exercise prescription was calculated as the number of exercise sessions successfully completed (i.e. participant completed the exercise session at the planned duration and intensity) divided by the number of planned sessions attended. Non- adherence was defined as any exercise session requiring exercise dose modification of either the planned exercise duration and/or intensity.
Cardiopulmonary function. An incremental physician-supervised CPET with 12-lead ECG monitoring (Mac® 5000, GE Healthcare) was performed on an electronically-braked cycle ergometer (Ergoline, Ergoselect 100, Bitz, Germany) with expired gas analysis (ParvoMedics TrueOne® 2400, Sandy, UT, USA) to determine cardiopulmonary function (peak oxygen consumption; VO2peak) according to published guidelines [Citation14,Citation15]. Preceding exercise, three minutes of resting metabolic data was collected before participants began cycling at 20 Watts. Workloads were then increased 5–20 Watts/min until volitional exhaustion or symptom-limitation. Workload increments were determined by patient cardiopulmonary response to exercise during the first minute of the CPET and were standardized at baseline and post-intervention. During exercise, heart rate and rhythm and SpO2 were monitored continuously using a 12-lead ECG and pulse oximetry (BCI, Hand-Held Pulse Oximeter, Waukesha, WN, USA). Rating of perceived exertion was evaluated using the Borg Scale and blood pressure was measured non-invasively by manual auscultatory sphygmomanometery every two minutes. A CPET was considered ‘peak’ if two of the three following criteria were met: 1) maximal predicted heart rate; 2) respiratory exchange ratio of ≥ 1.10; and/or 3) volitional exhaustion.
Indications for terminating the exercise test included: 1) chest pain; 2) ischemic ECG changes (ST segment depression or elevation ≥ 0.1 mV); 3) abnormal blood pressure response (> 250 mmHg systolic; > 120 mmHg diastolic; drop in systolic pressure > 20 mmHg); SpO2 ≤ 85%; and 4) dizziness and/or nausea. Criteria for a positive exercise test included 0.1 mV deviation of ST segment horizontal or away from the baseline isoelectric line at 60 ms following the end of the QRS complex. ST segment changes toward the isoelectric line were not considered positive, regardless of the magnitude of change [Citation16].
Cardiac function. Two-dimensional transthoracic echocardiographic images using standard views were obtained with a Vevo 770 High-Resolution Imaging System equipped with a 30-MHz transducer (RMV-716; VisualSonics, Toronto, Canada) performed and averaged over three cardiac cycles according to American Society of Echocardiography guidelines [Citation17].
Patient-reported outcomes. QOL was assessed by the Functional Assessment of Cancer Therapy-Breast (FACTB) scale [Citation18]. The FACTB contains subscales for physical (7-items), functional (7-items), emotional (6-items), and social (7-items) well-being. The four subscales were summed to obtain the FACT-General (FACT-G) score (all 27-items) plus a breast cancer subscale (9 items) (FACT-B). The 13-item Fatigue Scale (FS) of the Functional Assessment Chronic Illness Therapy (FACIT) was utilized to assess fatigue [Citation19].
Clinical characteristics and exercise behavior. Clinical characteristics were assessed via medical chart review. Exercise behavior performed outside of the clinical trial was assessed by the Godin Leisure Time Exercise Questionnaire [Citation20] at baseline and post-intervention.
Statistical considerations
Ten patients/group provided 80% power to detect a mean 2.6 ml/kg/min between group improvement in VO2peak from baseline to post-intervention, assuming a SD of 6.10 ml/kg/min and a one-sided alpha of 0.10. It is currently not known whether a between difference improvement of 2.6 ml/kg/min is clinically important in women undergoing neoadjuvant for early breast cancer. Analysis of covariance was used to compare changes in cardiopulmonary function and PROs, with baseline value as the covariate. Model residuals were examined to confirm normality assumptions. Within each group, a paired t-test was used to assess changes from baseline for each of these outcomes. Fisher's exact tests and χ2-tests were used to examine between groups differences in the overall proportion of patients experiencing treatment-related AEs (e.g. nausea, pain, arthralgia); outcomes were categorically coded (n = 0/no or n = 1/yes) for the statistical analyses. A mixed-model repeated measures analysis of variance was used to compare between groups differences over time for CBC profiles. All efficacy outcomes were assessed under the intention-to-treat principle. A two-sided significance level of 0.05 was used for all statistical tests. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
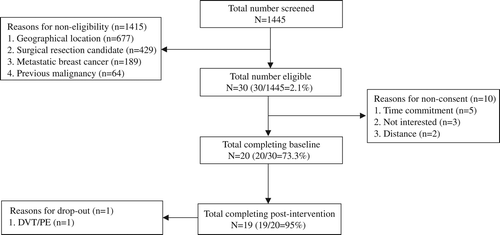
The study flow is presented in . Participant recruitment took place between March 2007 and January 2010. During the study period, 1445 patients attended a patient consultation with the Breast Tumor Group at DUMC. Of these, 30 (30/1445 = 2.1%) met inclusion criteria and 20 (20/30 = 73%) agreed to participate. The groups were balanced on all study outcomes at baseline (). Of these, 19 (19 of 20, 95%) completed all study procedures. One patient was lost to follow- up due to the development of deep vein thrombus (DVT) and pulmonary embolism (PE) following randomization. There were no significant changes in exercise behavior performed outside of the clinical trial in either group (p > 0.05).
Table I. Characteristics of participants.
Safety data
Cardiopulmonary exercise test abnormalities and adverse events. No positive tests were observed in either group. Two patients within the AC + AET group presented with resting ECG abnormalities (e.g. left axis deviation and left ventricular hypertrophy) at baseline and post-intervention. Three patients within the AC group presented with resting sinus tachycardia (i.e. resting heart rate > 100 bpm) at post-intervention. Infrequent, non-significant exercise-induced premature ventricular contractions (PVCs) were noted in both groups at baseline and post- intervention. Three non-life threatening/non-ECG-related AEs occurred during baseline exercise testing, which resulted in prematurely stopping the tests due to exercise-induced oxygen desaturation (SpO2 = 84%), anxiety attack, and dizziness. All symptoms/signs resolved promptly upon cessation of exercise and did not preclude study participation.
Treatment-related adverse events. There were no significant between group differences for any of the clinician-documented treatment-related events (p > 0.05). Hemoglobin levels significantly decreased in both groups (all p's < 0.05); the decline was attenuated in the AC + AET (p = 0.06). Platelet count significantly increased (p = 0.047) whereas hematocrit significantly decreased (p = 0.005) in the AC + AET group only. A total of five patients (n = 4 AC + AET group; n = 1 AC group) experienced therapy-related AEs that required medical management. Four patients in the AC + AET group were evaluated for newly diagnosed AEs including persistent tachycardia, diverticulosis, urinary tract infection (UTI), diabetes mellitus, upper respiration tract infection, hemorrhoids, DVT, and PE (more than one event was observed in the same patient). The same patient experienced the DVT and PE; this patient was censored since DVT/PE is an aerobic training contraindication. One patient in the AC group developed shingles secondary to varicella zoster infection.
Aerobic training-related adverse events. A total of one aerobic training-related AE was observed consisting of unexplained leg pain that quickly resolved following exercise cessation.
Aerobic training attendance and prescription adherence. Overall attendance to planned exercise sessions was 82% (296 attended/360 prescribed; range, 0–100%). Overall adherence to the planned exercise prescription was 66% (194 adhered sessions/296 attended). As a result, 34% of planned sessions required dose modification. Specifically, 23% (68/296) of sessions required a reduction in exercise duration and/or intensity, whereas 11% (34/296) required an increase in exercise duration and/or intensity. Major reasons for dose reductions were nausea, tiredness/fatigue, and not feeling well; major reasons for dose escalation were per exercise trainer adjustment or patient request.
Efficacy data
Cardiopulmonary function. VO2peak increased by + 2.6 ± 3.5 ml/kg/min (+ 13.3%) in the AC+ AET group and decreased by 1.5 ± 2.2 ml/kg/min (-8.6%) in the AC group (mean difference + 4.1 ml/kg/min favoring AC + AET, p = 0.001; ). Differences between groups were also observed for absolute VO2peak, peak power output, and Oxygen pulse (all p's < 0.05; ). All echocardiograms were normal at baseline and post-intervention (). There were no significant differences within- or between group changes in any cardiac parameters over time.
Table 2. Efficacy of aerobic training on cardiopulmonary function.
Patient-reported outcomes. FACT-B increased 11.1 points in the AC + AET group compared to a 1.5 points decrease in the AC group (mean difference + 12.6 points, p = 0.685; ). There was a borderline significant increase in the FACT-G for the AC+ AET group (p = 0.088). A significant within-group increase in emotional well-being was observed in both groups (p's < 0.05) but no between group difference. For social well-being, there was a significant increase in the AC+ AET group (p = 0.033), whereas physical well-being significantly decreased in the AC group (p = 0.004) (). There were no significant differences between groups for self-reported exercise behavior (p's > 0.05).
Table III. Efficacy of aerobic training on patient-reported outcomes.
Discussion
The principal findings of this pilot study were that: 1) supervised aerobic training program incorporating high-intensity aerobic interval training is safe (and relatively well tolerated) adjunct therapy in women undergoing anthracycline-containing chemotherapy for operable breast cancer; 2) anthracycline-containing chemotherapy alone is associated with marked reductions in cardiopulmonary function; 3) aerobic training not only completely abrogates the detrimental impact of chemotherapy but causes significant improvements in cardiopulmonary function during concurrent neoadjuvant therapy; and 4) there were favorable improvements in several PROs. To our knowledge, this is the first trial to evaluate the role of aerobic training during neoadjuvant therapy and one of first to extensively review the risk-to-benefit ratio of moderate-to-high intensity aerobic training during cytotoxic therapy. Overall, our findings have important implications for the design and implementation of exercise-based rehabilitation programs in the oncology setting.
Structured exercise training is receiving increased attention as an important adjunct therapy for patients following a cancer diagnosis [Citation21]. In this context, systematic reviews and meta-analyses [Citation1,Citation2] conclude that exercise training is a safe therapy for cancer patients during and following cancer therapy. Despite this, only 14% of exercise – oncology studies reported assessing AEs during trial conduct [Citation5]. Furthermore, in the studies reporting AEs, few adopted standardized monitoring and/or reporting of AEs. As such, the safety of supervised aerobic training in the oncology setting remains inconclusive. Against this background, we examined a broad range of information including aerobic training- and treatment-related data to evaluate this question.
Overall, we observed a total of six AEs during trial conduct. We did observe numerically more treatment-related AEs in the AC + AET group, however, these events were not likely attributable to aerobic training. Of the observed AEs, only one (i.e. DVT/PE) was considered serious requiring hospitalization and aerobic training discontinuation; again, whether this was directly attributable to aerobic training is inconclusive. To supplement clinician-reported data, we also evaluated serial changes in the CBC panel, which is a well-established method to monitor patient health status/response to therapy. Overall, the changes in CBC markers were unremarkable in both groups. As expected, hemoglobin concentration did significantly decrease during chemotherapy, although this decline was attenuated in the AC + AET group, suggesting that aerobic training could partially attenuate therapy-induced anemia; further investigation is required in larger trials.
The low incidence of AEs in this trial is consistent with the conclusions of prior reviews and meta-analyses as well as that of the recently published ACSM cancer-specific exercise guidelines [Citation1,Citation2]. However, it is important to note that the vast majority of studies to date in the oncology setting have evaluated the efficacy (and safety) of aerobic training programs following standard exercise prescription guidelines [Citation2]. Studies evaluating the efficacy of exercise prescriptions incorporating high-intensity aerobic interval training, however, require separate consideration given the markedly higher ‘stress’ to the cardiopulmonary system and potential associated adverse consequences (e.g. sudden death, myocardial infarction). To our knowledge, several studies, [Citation9,Citation22] including work from our own group [Citation10,Citation11], have assessed the efficacy of exercise prescriptions incorporating high-intensity aerobic training; none of these studies were designed to primarily address safety nor do they report adopting standardized monitoring and/or reporting of AEs. A total of five exercise training-related AEs were reported across all five trials with no AEs requiring hospitalization or exercise discontinuation. Similarly, a retrospective review of 4846 unselected patients with coronary heart disease treated with exercise training incorporating high-intensity aerobic interval training in cardiac rehabilitation revealed that such training is associated with a low cardiovascular event rate [Citation23]. As such, based on current evidence, exercise prescriptions incorporating high-intensity training appear to be associated with a low incidence of AEs although further rigorous evaluation is required.
A noteworthy secondary finding of our study was that a CPET is a feasible and safe tool to assess peak exercise cardiopulmonary function in breast cancer patients both before and following the completion of neoadjuvant AC therapy. Specifically, 85% of patients were able to achieve the criteria of a peak test with a low incidence of exercise-induced ECG abnormalities and no life-threatening AEs. The low incidence of events corroborates that observed in other clinical populations. However, the lack of serious AEs during CPET in the present study is not completely unexpected given the strict eligibility criteria and small sample size. Thus, our findings are not generalizable to a broader, unscreened population of women with early-stage breast cancer. Indeed, the risk of CPET-related AEs in the oncology setting, as in other settings, is highly dependent on pre-existing comorbid disease and other patient-related characteristics [Citation14,Citation24]. For example, in prior work investigating the safety of CPET in 85 patients with advanced cancer, a total of three positive tests were observed while 26% and 44% of patients with advanced non-small cell lung cancer and metastatic breast cancer developed asymptomatic ST segment changes during the CPET [Citation25]. Of relevance, the Canadian Society for Exercise Physiology (CSEP) recently published the first pre-exercise testing/training screening recommendations for cancer patients [Citation5]. Based on these guidelines, patients treated with anthracycline-containing regimens are considered intermediate risk (of an exercise-related event) and require exercise testing with ECG monitoring. While no life-threatening AEs were observed during the CPET, the present results support this recommendation since exercise-induced ECG abnormalities were detected, which necessitates rigorous monitoring during testing and subsequent aerobic training sessions. Formal investigations of the safety of CPET and exercise training in the oncology setting are required.
An important complementary aspect to consider when evaluating safety of exercise training is tolerability of training. In the current trial, exercise attendance and adherence data was evaluated to provide additional information regarding the tolerability of moderate-to-high intensity aerobic training in this setting. The attendance rate was 82%, which is within the conventionally accepted rates and consistent with prior work [Citation26,Citation27]. The adherence rate was 66%. Courneya et al. [Citation26] reported attendance and adherence rates of 72% and approximately 95%, respectively, in women undergoing supervised moderate-intensity aerobic (i.e. 60–80% of baseline VO2peak) or resistance (i.e. 60–70% of estimated one-repetition maximum) training during standard adjuvant chemotherapy for early-stage breast cancer. The reasons for the divergent findings on adherence to the exercise prescription between our study and that of Courneya et al. are not known but may be related to study methodology, the different exercise intensities investigated (i.e. moderate intensity vs. moderate-to-high intensity) and definition of adherence. Nevertheless, prescriptions incorporating higher-intensity exercise training may not be feasible or tolerable for all patients undergoing primary neoadjuvant therapy. Ultimately, exercise prescriptions need to be individually tailored (based on CPET results and patient characteristics) and account for treatment/disease-related symptoms prior to and during sessions, exercise prescriptions will need to be modified in ‘real-time’ by qualified exercise personnel. To this end, exercise training trials in clinical settings classically report attendance rates to provide data on the tolerability of the intervention. However, such measures may need to be supplemented by close monitoring of adherence to fully evaluate the ability of patients to participate in and benefit from the intervention. While our results need to be confirmed, the current findings imply that exercise rehabilitation will need to be conducted in a supervised outpatient or inpatient setting (particularly during treatment) to enable ‘real-time’ monitoring and modification of exercise sessions.
Finally, there were significant improvements in several markers of cardiopulmonary function in the AC + AET group compared with the AC group. Specifically, we found a between group difference in VO2peak of 4.1 ml/kg/min favoring the AC + AET group. Gulati et al. [Citation28] found that the Framingham Risk Score-adjusted mortality risk decreased by 17% for every 3.5 ml/kg/min difference in exercise capacity among asymptomatic women, suggesting that the observed difference in VO2peak may be clinically meaningful. To our knowledge, this is one of the first studies to observe significant improvements in VO2peak in breast cancer patients receiving adjuvant chemotherapy. For example, Courneya et al. [Citation26] reported that supervised aerobic training was superior to usual care (chemotherapy only) for improving VO2peak in 242 operable breast cancer patients receiving standard adjuvant chemotherapy. Interestingly, aerobic training was associated with a non-significant improvement in VO2peak (+ 0.5 ml/kg/min) but completely abrogated the VO2peak decline observed in the usual care group. Similarly, in our study, AC alone was associated with an approximately 9% decline in VO2peak; VO2peak typically declines 10% every decade in healthy women indicating that 12 weeks of AC may cause a decade of ‘physiological aging’. Moreover, the VO2peak decline may not recover, even years following the cessation of primary therapy. For example, we found that despite ‘normal’ resting cardiac function (i.e. LVEF ≥ 50%), VO2peak was, on average, 22% below that of age-matched sedentary women in early breast cancer patients a mean of 27 months following the completion of primary adjuvant therapy [Citation29]. Together the data from the present study and findings from Courneya et al. highlight that without intervention, standard chemotherapy-containing adjuvant therapy causes significant impairments in cardiopulmonary function. Aerobic training was associated with improvements in select PROs. Our findings are consistent with previous reports indicating that the effects of aerobic training on QOL and other PROs during therapy are mixed [Citation1]. Heterogeneity in patient clinical characteristics, setting, treatment, and measurement tools are likely responsible for the equivocal findings.
This study does have limitations. The most important limitation is our small sample size. The neoadjuvant setting provides many benefits for testing the effects of exercise training but selecting a single regimen at a center for purposes of homogeneity limits the potential for accrual. Larger trials would require evaluation in the adjuvant setting, involving multiple centers, and/or accrual of patients on multiple therapeutic regimens. Relatedly, given our relatively stringent eligibility criteria together with the requirement of attending exercise sessions in a supervised setting, only 2% of total screened patients were eligible for the trial (47% of potential participants were excluded due to geographical distance from DUMC). Although our data do provide support for a larger study, the generalizability of our findings to the larger population of breast cancer patients receiving adjuvant chemotherapy is limited. Finally, our intervention period (12 weeks) was short; trials investigating the safety/efficacy of exercise across the entire duration of primary neoadjuvant therapy are required to fully address this question.
In summary, aerobic training prescriptions incorporating high-intensity aerobic interval training when conducted with one-on-one supervision is a safe adjunct therapy associated with improvements in cardiopulmonary function and select PROs during neoadjuvant chemotherapy. Larger trials further investigating the safety and efficacy of both moderate- and high-intensity exercise training prescriptions are warranted. Such trials are essential to fully evaluate the risk-to-benefit ratio of exercise training during cytotoxic therapy. Nevertheless, given the demonstrated safety and superior cardiopulmonary benefit of high-intensity aerobic interval training in patients with coronary heart disease, our findings suggest that incorporation of high-intensity aerobic training into exercise-based rehabilitation programs may be of benefit in early-stage breast cancer patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by the United States Department of Defense Breast Cancer Research Program of the Office of the Congressionally Directed Medical Research Programs – Ideas Award and funds from George and Susan Beischer (LWJ). LWJ is supported by research grants from the National Cancer Institute (CA143254, CA142566, CA138634, CA133895, CA164751).
References
- Schmitz KH, Courneya KS, Matthews C, Demark- Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42: 1409–26.
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. Cancer J Clin 2012; 62:243–74.
- Hayes SC, Spence RR, Galvao DA, Newton RU. Australian Association for Exercise and Sport Science position stand: Optimizing cancer outcomes through exercise. J Sci Med Sport 2009;12:428–34.
- van den Berg JP, Velthuis MJ ,Gijsen BC, Lindeman E, van der Pol MA, Hillen HF. [Guideline “Cancer rehabilitation”]. Ned Tijdschr Geneeskd 2011;155:A4104.
- Jones LW. Evidence-based risk assessment and recommendations for physical activity clearance: Cancer (1) (1) This paper is one of a selection of papers published in this Special Issue, entitled Evidence-based risk assessment and recommendations for physical activity clearance, and has undergone the Journal’s usual peer review process. Appl Physiol Nutr Metab 2011;36(Suppl 1):S101–12.
- Munk PS, Butt N, Larsen AI. High-intensity interval exercise training improves heart rate variability in patients following percutaneous coronary intervention for angina pectoris. Int J Cardiol 2010;145:312–4.
- Rognmo O, Moholdt T, Bakken H, Hole T, Molstad P, Myhr NE, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012;126:1436–40.
- Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation 2008;118:346–54.
- MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res 1989;38:348–51.
- Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer 2007;110:590–8.
- Courneya KS, Jones LW, Peddle CJ, Sellar CM, Reiman T, Joy AA, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: A randomized controlled trial. Oncologist 2008;13:1012–20.
- Carter SK, Blum RH. New chemotherapeutic agents – bleomycin and adriamycin. Cancer J Clin 1974;24: 322–31.
- Brolinson PG, Elliott D. Exercise and the immune system. Clin Sports Med 2007;26:311–9.
- ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–77.
- Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol 2008;9:757–65.
- Sanchis J, Bodi V, Nunez J, Bertomeu-Gonzalez V, Gomez C, Consuegra L, et al. Usefulness of early exercise testing and clinical risk score for prognostic evaluation in chest pain units without preexisting evidence of myocardial ischemia. Am J Cardiol 2006;97:633–5.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63.
- Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997;15:974–86.
- Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol 1997;34(3 Suppl 2):13–9.
- Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: A concurrent validity study. Can J Public Health 1986;77:359–62.
- Jones LW, Peppercorn J. Exercise research: Early promise warrants further investment. Lancet Oncol 2010;11:408–10.
- Quist M, Rorth M, Langer S, Jones LW, Laursen JH, Pappot H, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: A pilot study. Lung Cancer 2012;75:203–8.
- Kemi OJ, Wisloff U. High-intensity aerobic exercise training improves the heart in health and disease. J Cardiopulm Rehabil Prev 2010;30:2–11.
- Skalski J, Allison TG, Miller TD. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation 2012;126:2465–72.
- Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer 2007;55:225–32.
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–404.
- Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J Clin Oncol 2010;28: 340–7.
- Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003;108:1554–9.
- Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 2012;30:2530–7.