Abstract
Background. Cancer of unknown primary (CUP) carries a dismal prognosis due to the two hallmarks of 1) being metastatic with 2) no known specific treatment. An organ-labeled diagnosis of cancer should therefore be sought. In this study, we have analyzed population-based incidence and survival data of CUP over the latest 40 years. Material and methods. Complete national data on 23 004 CUP-patients from the Cancer Registry of Norway sampled from 1971 to 2010 are presented, with absolute and age-adjusted incidence rates correlated to the total cancer incidence. One-year relative survival rates were calculated. Results. The incidence of CUP increased both in absolute numbers and as a fraction of total cancer incidence during the first half of the period. There has been a substantial decrease in incidence over the latest 20 years, now being responsible of only 1.7% and 1.2% of the total cancer incidence in females and males, respectively, with an age-adjusted incidence rate of 3.5 and 3.8, respectively. The one-year relative survival rate has increased and was slightly below 20% for both sexes in 2010. Conclusion. Better diagnostics, both radiological and pathological, is probably responsible for a substantially lower incidence. Improved treatment for cancers in general also benefits the CUP-group.
The diagnosis cancer of unknown primary (CUP) is by definition metastatic, and invariably confers a dismal prognosis [Citation1]. Furthermore, due to its unknown organ-specified origin the condition is hampered with an even worse prognosis as disease-specific treatment is unavailable and management must be based on assumptions on aggressiveness and probabilities of origin [Citation2]. The diagnostic work-up should therefore be thorough, and efforts made to improve specification. This confers especially to radiological and histopathological examinations [Citation3,Citation4].
Current literature estimates the incidence of CUP to 3–5% of all cancer diagnoses, but some reports have pointed to a certain reduction of incidence during the latest years [Citation5]. In a recent publication from Sweden a reduction in incidence rates from approximately 7–6 per 100 000 from 1987–2008 was shown [Citation6]. The one-year survival is in various reports reported below 20%, but depends on prognostic stratification [Citation7,Citation8].
In this study we have population-based data of CUP incidence and survival from Norway, in the period from 1971 to 2010. During this period, the diagnostic possibilities were improved, especially due to increased use of CT-scanning from the mid-1970s, PET-scannings from the early 2000s, and increased use of immunohistochemistry. Here we report incidence and survival data on a national level during this period of improved diagnostic possibilities.
Material and methods
Norway, with a population of 5 million, has a universal public health service financed by taxation and a national insurance scheme accessible to all residents, independent of social or economic status. In the period studied, the proportion of citizens of non-western origin fluctuated from 1.3% to 4.9%. In our data set no subset analysis was conducted based on race, socioeconomic status or geography.
Data collection
Since 1952 all malignant neoplasms have been reported to the Cancer Registry of Norway. In addition, copies of cytology, biopsy and autopsy reports are submitted from pathology laboratories as well as death certificate reports from the Cause of Death Register run by the National Statistics Bureau, Statistics Norway. Since 1998 all hospitals have filed discharge summaries electronically to the registry. The system of reporting to the Cancer Registry of Norway was evaluated in 2009, and overall completeness of reporting was estimated at 98.8% [Citation9].
For the current study, we have included ICD-10 diagnose code C39, C76 and C80, and for previous periods similar diagnoses have been included depending on the coding system used.
Patient follow-up
Mortality updates are conducted routinely by the Cancer Registry of Norway through linkage with the Cause of Death Register. In survival analyses, the primary outcome was survival following a diagnosis of CUP. Follow-up was defined as the time from CUP diagnosis to death as reported from the Cause of Death Register or to the last date of data submission for patients who were still alive, whichever came first.
Statistical methods
We divided the study period into calendar years. Incidence data are presented as absolute numbers, and age-adjusted rates by the direct method to the standard world population. We calculated one-year male and female relative survival for individuals diagnosed through successive five year calendar periods. We estimated relative survival for men and women using the method of Hakulinen, where the survival time of the matched individual is censored at the same survival time as that of the patient with cancer [Citation10]. Relative survival adjusts for competing causes of death expected for persons of the same sex, age and calendar year of investigation (the ratio of observed survival in a population to the expected survival rate); hence cause of death information is not required.
Results
Incidence
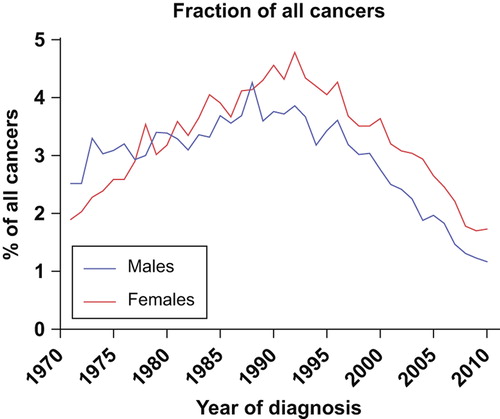
A total of 23 004 cases of cancers of unknown primary are registered by the Cancer Registry of Norway in the 40-year period 1971–2010. The age-adjusted incidence rate per 100 000 in women increased from 2.8 in 1971–1975 to 6.2 in 1991– 1995, and decreased to 3.5 in 2006–2010. In men the rates increased from 4.9 in 1971–1975 to a peak of 7.9 in 1991–1995, decreasing to 3.8 in the latest five-year period (). The incidence of CUP in absolute numbers increased steadily during the first 20 years, from 254 patients in 1971 to 830 patients in 1992. In the latter part of the period, there has been an approximately 50% decrease, to 405 new cases in 2010 (). This corresponds to an average absolute annual decrease over the latest 20 years of 3.4% in men and 2.8% in women, whereas the age-adjusted annual decrease has been 3.3% and 2.3%, respectively, in the same period. In the first 30-years of the period, the incidence in males was significantly higher than in women (p = 0.028), but this has become insignificant during the latest 10 years.
Figure 1. The age-adjusted incidence rates for CUP increased until 1990, but has shown a substantial decrease over the latest 20 years.
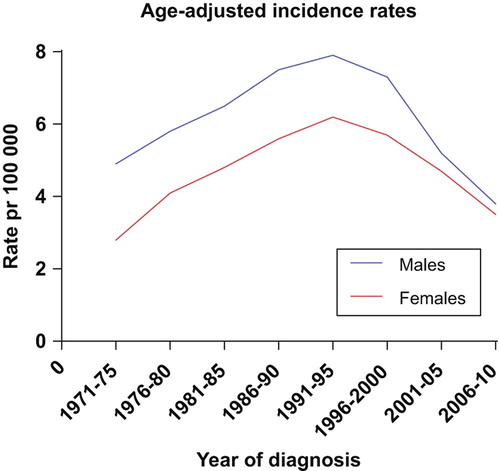
Figure 2. The incidence of CUP in absolute numbers has followed a different curve than the general cancer incidence in Norway.
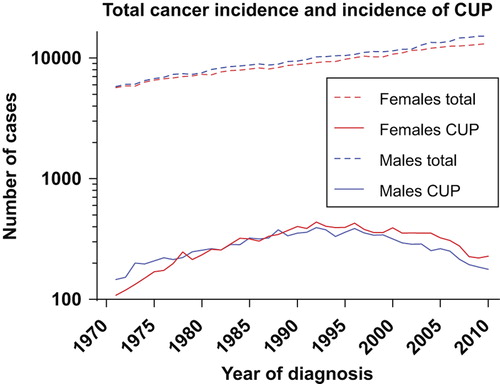
The total number of cancer cases has increased steadily over the 40-year period in Norway, from 5674 cases in females and 5793 in males in 1971, to 13 161 and 15 110 cases, respectively, in 2010 (). The CUP-fraction of the total numbers thus has decreased substantially, accounting for only 1.73% and 1.17% of all cases for females and males, respectively, in 2010 (). The highest fractions were reached in 1992 for females (4.78%) and 1988 for males (4.26%).
Survival
The one-year survival has fluctuated around 10% throughout the period, but the latest five years there has been an upward trend for both sexes, being in 2010 19.4% for males (95% CI 13.2–26.6) and 18.3% (95% CI 12.9–24.6) for females (Supplementary Figure 1, available online at http//www.informa healthcare.com/doi/abs/10.3109/15622975.2013.783230). The median survival is slightly higher for women than for men, as is seen for most cancer types, but is generally low, around 2–3 months. A slight improvement has been seen in the latest years, most evident for men (Supplementary Figure 2, available online at http//www.informahealthcare.com/doi/abs/10.3109/15622975.2013.783230).
Discussion
This study confirms and extends the knowledge on the decrease in incidence of cancers of unknown primary over the latest 20 years [Citation11]. The incidence in males was substantially higher than in females until the last 10 years, but the decrease rate has since been larger in men. The incidence peak for both sexes was reached around 1990. The decrease might be explained by the fact that the diagnostic armamentarium has been gradually improved, as more endoscopies and CT scans have been performed and also an increased pathological accuracy has evolved, in particular, due to increased use of immunohistochemistry and other tests including molecular genotyping.
The one-year survival has during the recent years improved from around 10%, now approaching 20% for both sexes. This may have several causes. The diagnostics have improved since 1970, with CT's being available from the mid-1970s, MRI from the 1980s and PET-scanners during the latest 10 years. Cancers therefore are generally diagnosed in earlier stages and with better accuracy in recent years [Citation12]. Furthermore, the advent of diversified immunohistochemistry as well as other molecular classifications (although not widely employed clinically), has undoubtedly increased the diagnostic specification [Citation13]. Thereby more patients are probably given a therapy-directing tentative, although not definitive, diagnosis that is more in concordance with the true origin of the tumor.
Better stratification of CUP patients into favorable or unfavorable subsets, and an improved treatment algorithm – e.g. axillary lymph-node only patients are treated aggressively as breast cancer – might itself have resulted in better survival results lately [Citation14].
The advent of improved chemotherapy might have contributed to the increased one-year survival observed in the later years, even though the chemotherapy-effect has been questioned [Citation8]. Finally, the improved survival might rely on a reduced fraction of the most unfavorable diagnoses in the CUP-category. Aggressive tumors as pancreatic cancers and lung cancer might be represented to a less extent, due to improved radiological diagnostics. The substantial increase of these, especially lung cancer, in Norway might corroborate this hypothesis [Citation15]. Interestingly, the reduction of CUP, also among women, is seen in a period where there has been a dramatic increase of smoking-related cancers among women (particularly adenocarcinomas of the lung) following the increased prevalence of female daily-smokers which only over the recent few years has shown a declining trend [Citation15].
The fact that the one-year survival has increased whereas the median survival has improved to a less extent may indicate and confirm that there is a subgroup of patients that has a prolonged survival, but that the majority still has a dismal prognosis.
In conclusion, we have shown a substantial decline in the frequency of CUP on a national level, and also a better prognosis. Still the disease affects a large number of patients, and the diagnosis is generally dismal. Struggles should therefore be made to reduce the frequency further, and to improve the survival for one of the hitherto most fatal of all malignancies.
Supplementary Figure 2
Download PDF (318.4 KB)Acknowledgements
The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred. Funding assistance was given by the South-Eastern Norway Regional Health Authority. The authors report no conflicts of interest.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet 2012;379:1428–35.
- Pavlidis N. Optimal therapeutic management of patients with distinct clinicopathological cancer of unknown primary subsets. Ann Oncol 2012;23(Suppl 10):x282–5.
- Taylor MB, Bromham NR, Arnold SE. Carcinoma of unknown primary: Key radiological issues from the recent National Institute for Health and Clinical Excellence guidelines. Br J Radiol 2012;85:661–71.
- Oien KA, Dennis JL. Diagnostic work-up of carcinoma of unknown primary: From immunohistochemistry to molecular profiling. Ann Oncol 2012;23(Suppl 10):x271–7.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29.
- Bevier M, Sundquist J, Hemminki K. Incidence of cancer of unknown primary in Sweden: Analysis by location of metastasis. Eur J Cancer Prev 2012;21:596–601.
- Hemminki K, Bevier M, Hemminki A, Sundquist J. Survival in cancer of unknown primary site: Population-based analysis by site and histology. Ann Oncol 2012;23:1854–63.
- Golfinopoulos V, Pentheroudakis G, Salanti G, Nearchou AD, Ioannidis JP, Pavlidis N. Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: Multiple-treatments meta-analysis. Cancer Treat Rev 2009;35:570–3.
- Larsen IK, Smastuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the Cancer Registry of Norway: An overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31.
- Hakulinen T. Cancer survival corrected for heterogeneity in patient withdrawal. Biometrics 1982;38:933–42.
- Randén M, Rutqvist LE, Johansson H. Cancer patients without a known primary: Incidence and survival trends in Sweden 1960–2007. Acta Oncol 2009;48:915–20.
- Jerusalem G, Rorive A, Ancion G, Hustinx R, Fillet G. Diagnostic and therapeutic management of carcinoma of unknown primary: Radio-imaging investigations. Ann Oncol 2006;17(Suppl 10):x168–76.
- Bahrami A, Truong LD, Ro JY. Undifferentiated tumor: True identity by immunohistochemistry. Arch Pathol Lab Med 2008;132:326–48.
- Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G, Group EGW. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow- up. Ann Oncol 2011;22(Suppl 6):vi64–8.
- Sagerup CM, Småstuen M, Johannesen TB, Helland Å, Brustugun OT. Sex-specific trends in lung cancer incidence and survival: A population study of 40,118 cases. Thorax 2011;66:301–7.