To the Editor,
Uveal melanoma arises from melanocytes that reside in the iris, ciliary body or choroid of the eye. Local treatment can be divided into ‘radical’ enucleation and ‘conservative’ treatment. About 50% of patients develop metastasized disease and in up to 95% of these cases the liver is affected, due to the absence of lymphoid structures in the uvea. Once metastasized to the liver, surgical resection may be beneficial for small lesions, but less than 9% of patients fall into this category [Citation1].
The blockade of cytotoxic T lymphocyte- associated antigen-4 (CTLA4) by ipilimumab has become standard for pretreated patients with cutaneous melanoma based on a randomized phase III study [Citation2]. This drug significantly improved the overall survival resulting in 20–25% of the patients still being alive after more than two years.
Due to its distinct biological and clinical nature (fast progression) uveal melanoma patients are often excluded from melanoma studies. Uveal melanoma patients have been allowed to be included in ipilimumab expanded access programs, in which some clinical activity has been described [Citation3–6].
In our study, 22 pretreated metastatic uveal melanoma patients were treated homogenously with 3 mg/kg ipilimumab in the named patient program (NPP) by the Dutch immunotherapy working group (WIN-O) in The Netherlands. We describe here the toxicity and efficacy of ipilimumab at 3 mg/kg in a real world patient cohort of uveal melanoma patients.
Methods
Patients
Patients were treated by the Dutch immunotherapy working group (WIN-O) in a NPP of ipilimumab (NCT00495066) in which uveal melanoma patients were allowed to be included. Patients had to have unresectable, metastatic uveal melanoma (with or without brain metastases) and were required to have received at least one prior treatment regimen for metastatic disease. They had to be at least 16 years of age with a WHO performance status of 0, 1, or 2. A 28-day interval since the last treatment was required before inclusion. Evaluable patients that had given their written informed consent underwent radiologic evaluation of their tumor burden at baseline and at 12 weeks after their first ipilimumab course. The treatment protocol was approved by the local medical ethical committees.
Treatment
Ipilimumab was administered at 3 mg/kg in week 1, 4, 7 and 10. Prior to every infusion, hemoglobin, leucocytes and differentiation, platelets, liver function, renal function, thyroid and adrenal function were assessed for safety reasons and monitoring of toxicity. Immune-related adverse events (IrAEs) were scored using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Response and survival evaluation
At baseline and after four courses of ipilimumab at week 12, a computed tomography (CT) scan was made to evaluate the tumor response. We used the following radiological scoring systems; immune-related response criteria (irRC) and RECIST version 1.1. The response rates were termed as partial remission (PR) and complete remission (CR). BOR was also assessed using irRC to capture delayed anti-tumor responses often observed with immunotherapy. Clinical benefit was defined as the response proportion of patients plus SD lasting longer than 24 weeks. Estimates of OS and PFS were obtained using the Kaplan-Meier method.
Data-analysis
Data were retrospectively collected from all Dutch centers organized in the Dutch immunotherapy working group (WIN-O) participating in the Dutch expanded access program and having treated uvela melanoma patients (see also coauthors affiliations). Patients’ data were retrospectively collected into a predefined SPSS database by each center individually. Descriptive statistics were performed using SPSS statistical software (version 17.0 for Windows, SPSS, Chicago, USA). The final data were graphed and analyzed using GraphPad Prism Version 5.0.
Results
Twenty-two metastatic uveal melanoma patients were treated in an NPP, which was open in The Netherlands from May 2010 until August 2011. The patient characteristics of this cohort are described in (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.786839). Median follow-up was 177 days (6.3 months). Twelve patients (55%) completed the four infusions of ipilimumab. Of the remaining 10 patients, nine had to discontinue treatment because of clinical deterioration due to disease progression (two of them died) and one because of severe adverse events ().
Figure 1. OS and PFS of uveal melanoma patients treated with ipilimumab 3 mg/kg. All uveal melanoma patients treated in the Dutch expanded access program were evaluated retrospectively for OS (red, ) and PFS (blue, ) All 22 patients were included for PFS analysis, and the patients not evaluable at week 12 were defined to be progressive at the date of clinical deterioration. The detailed follow-up of the patients during treatment is shown in .
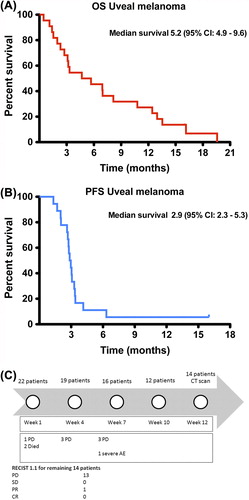
In the response to treatment is described. Of the 22 patients who received at least one ipilimumab infusion, 13 patients showed progressive disease (PD) and one patient had a PR. There was no SD or CR achieved according to RECIST 1.1. Eight patients were not evaluable (NE). Following irRC there were 12 patients with PD, one with SD, one with PR and no CRs.
Table I. Response to treatment.
At the time of manuscript preparation one patient (4.5%) was still alive with ongoing SD (+ 16 months). The patient observing a PR was eligible for ipilimumab re-induction due to disease progression seven months after ipilimumab initiation. Unfortunately, the re-induction did not result in a renewed response.
The OS and PFS curves of our 22 patients are depicted in . The Kaplan-Meier analyses show a median PFS of 2.9 months. The median OS was 5.2 months with a one-year survival of 27%.
As shown in (Supplementary Table II to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.786839) most adverse events were immune-related. Here, we only describe the grade 3 irAEs, as grade 1 or 2 was not considered clinically relevant. Grade 3 colitis was seen in two patients. One patient developed grade 3 hepatitis. All patients received corticosteroid treatment (1 mg/kg predisolon) after which irAEs quickly resolved.
Discussion
In our study, 22 M1c uveal melanoma patients were treated by the Dutch immunotherapy working group (WIN-O) in an ipilimumab NPP in The Netherlands. Only 12 patients (55%) completed the treatment course consisting of four infusions of ipilimumab at the dose of 3 mg/kg. Within the cohort of the 22 patients, only one patient had a PR according to RECIST and another patient had SD according to irRC.
In another recently published study performed by Danielli et al., nine of 13 patients (69%) completed the course of four infusions and two patients showed SD that remained until week 36 [Citation4]. Median OS was 36 weeks (9 months), in contrast to 21 weeks (5.2 months) in our cohort.
Three other, so far unpublished, retrospective analyses have evaluated the efficacy of ipilimumab in uveal melanoma patients. A single center analysis of 20 uveal melanoma patients treated at the Memorial Sloan-Kettering Cancer Center observed within a group of 20 patients that received a median of four infusions of ipilimumab (20%) two PRs (one at week 12 and one at week 24) and seven SD. This resulted in a median survival of 8.6 months (95% CI 3.5-NR), with two ongoing PRs (3 + yrs and 24 + wks) [Citation3]. The other expanded access programs, the Italian and the US, observed a one-year OS rate of 32% and 34%, respectively, which were comparable to the one-year OS rate observed in our study (27%) [Citation5,Citation6].
Furthermore, initial phase I studies indicated a correlation between the presence of grade 3-4 irAEs and response [Citation7], that was not confirmed in the phase III studies [Citation2,Citation8]. Similarly, no such correlation was found in our analysis.
In conclusion, our retrospective analysis from the Dutch expanded access program indicates limited clinical activity of ipilimumab in pretreated patients with metastatic uveal melanoma at a dose of 3 mg/kg. Currently, two single-arm phase II clinical trials are testing ipilimumab in uveal melanoma patients (www.clinicaltrials.gov Identifier: NCT01355120 and NCT01034787). In addition a phase Ib/II study exploring the combination of ipilimumab with radiofrequency ablation (RFA) in uveal melanoma patients has been started recently at the Netherlands Cancer Institute (NKI-AVL), Amsterdam (www. trialregister.nl Identifier: NTR3488). Intensive patient characterization and biomarker research in these studies will hopefully be able to identify predictive factors for response and survival to targeted therapy and immunotherapy in metastatic uveal melanoma.
Supplementary Tables 1 and 2
Download PDF (611.7 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
A.J.M. Van Den Eertwegh, G.A.P. Hospers, E. Kapiteijn and C.U. Blank have received compensation for participation in advisory board meetings of BMS Netherlands C.U. Blank receives funding for an investigator initiated study from BMS
References
- Sato T, Han F, Yamamoto A. The biology and management of uveal melanoma. Curr Oncol Rep 2008;10:431–8.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med 2010;363:711–23.
- Khan SA, Callahan MK, Postow M, Chapman P, Schwartz G, Dickson M, et al. Ipilimumab in the treatement of uveal melanoma: The Memorial Sloan-Kettering Cancer Center experience. Abstract #8549, ASCO Annual Meeting. 2012.
- Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: Safety and clinical efficacy. Cancer Immunol Immunother 2012;61:41–8.
- Maio M, Sileni VC, Pilla L, Nicoletti SVL, Di Guardo L, Queirolo P, et al. Efficacy and safety of ipilimumab in patients with pretreated, ocular melanoma: Experience from Italian clinics participating in the European Expanded Access Programme. Abstract #1133P, ESMO Annual Meeting. 2012.
- Lawrence D, McDermott D, Hamid O, Weber J, Wolchok J, Richard J, et al. Treatment of patients (pts) with stage III or IV melanoma on an ipilimumab (Ipi) expanded access program (EAP): Results for 3mg/kg cohort. Poster, SMR meeting. 2012.
- Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: A phase I/II study. Ann Surg Oncol 2005;12:1005–16.
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med 2011;364:2517–26.