Abstract
Background. To present a new method that determines an optimised IGRT couch correction vector from a displacement vector field (DVF). The DVF is computed by a deformable image registration (DIR) method. The proposed method can improve the quality of volume-of-interest (VOI) alignment in image guided radiation therapy (IGRT), and can serve as a decision-making aid for re-planning. Material and methods. The proposed method was demonstrated using the CT data sets of 11 head-and-neck cancer patients with daily kilovoltage control-CTs. A DVF was computed for each control-CT using a DIR method. The DVF was used for voxel tracking and re-contouring of the VOIs in the control-CTs. Then a rigid body transformation, which could be used as couch correction vector, was optimised. The aim of the optimisation process was to find a vector and rotations that map the deformed VOIs into a specified territory. This territory was defined by a margin extension of the VOIs at the time of the planning process. Within this extension, VOI motion and deformation was tolerated. The objective function in the optimisation process was the sum of all volume fractions outside the defined territories. Results. The proposed method was able to find a correction vector, which resulted in a coverage of the target volumes of at least 98% in 52.3% of all fractions. In contrast, a standard IGRT correction using a rigid registration method only fulfilled this criterion in 22.6% of all fractions. The optimisation process took an average of 1.5 minutes per fraction. Conclusion. The knowledge of the deformation of the anatomy allows the determination of an optimised rigid correction vector using our method. The method ensures controlled mapping of the VOIs despite small deformations. If no optimised vector can be determined, re-planning should be considered. Thus, our method can also serve as a decision-making aid for re-planning.
During radiation therapy inter-fractional anatomical changes occur. Deformable image registration (DIR) methods allow the detection and extraction of such changes from tomographical image scans. DIRs generate displacement vector fields (DVFs) which describe setup variations and deformations in control-CTs in respect to planning-CTs.
The main field of application of DVFs in clinical routine is the adaptation of the contours of volumes-of-interest (VOIs) to their new position on the control-CTs [Citation1–4], which can speed up the re- planning process. However, daily re-planning is time and effort consuming and not implemented in most radiation oncology departments. To at least correct for the systematic patient positioning variations, image guided radiation therapy (IGRT) is used routinely in clinical practice. This positioning correction is usually derived from a rigid registration method resulting in parameters for a shift of the treatment couch. The rigid registration can be limited to regions including different image content, which might result in different couch shifts [Citation5–7]. It is difficult for the treating physician to anticipate how the region for registration will impact the resulting tumour and organ alignment.
In this proof-of-principle study we introduce a new field of application for DVFs. We present a method that uses a DVF to optimise a three-dimensional (3D) treatment couch shift. This shift is determined by accounting for motions and deformations of structures within previously defined margin-extended volumes.
Additionally, we analysed the method to see if it can also be used to establish an intervention threshold for re-planning. If no optimised correction vector can be determined that allows mapping of the VOIs within the defined volume extension, deformations cannot be compensated sufficiently by a rigid couch shift. Therefore (online) re-planning should be considered. Thus, our approach also intends to reduce the efforts and costs of more complex correction strategies, such as online plan modification, at each treatment fraction.
Material and methods
Patients and target volume definitions
The proposed method was demonstrated using the CT data sets of 11 consecutively treated HNC patients that received daily kilovoltage control-CTs (mean 29.7 control-CTs/patient). All patients were treated with a linear accelerator combined with an in-room, single-slice spiral CT-scanner. A total of 327 (28–32 per patient) CT-scans (0.98 × 0.98 × 3.0 mm3 or 0.98 × 0.98 × 2.0 mm3) were evaluated. All patients received postoperative chemoradiation and were immobilised with a head mask and a vacuum pillow [Citation8].
Two CTVs were delineated: A therapeutic CTV (tCTV), which included the pre-surgical gross tumour volume, and a prophylactic CTV (pCTV), which included the supraclavicular and cervical lymph nodes. The tCTV was extended by a CTV- to-PTV margin of 3 mm to define the tPTV [Citation9]. Due to its extension and location, the larger pCTV was expected to be more prone to deformations than the tCTV. Thus, the pCTV was extended by a 5 mm CTV-to-PTV margin to define the pPTV. For IMRT treatment planning, a planning risk volume (PRV) surrounding the spinal cord was constructed by adding a 5 mm margin to the cord.
Deformation analysis
An automatic DIR method based on a template matching technique was used [Citation10]. No additional rigid pre-registration step was necessary, since the image scans were already correlated by external marker alignment. The main idea of the fully automated DIR method consisted of three steps: 1) Selection of small rigid templates (40 × 40× 40 mm3) distributed along the planning-CT scan. These templates included high contrast image structures, often bony anatomy, but also included distinctive soft tissue structures in kV-CTs; 2) Independent rigid optimisation of the position of the templates in respect to the control-CT scan using cross-correlation as similarity measure; and 3) Interpolation of the DVF using the resulting correspondences as supporting points for a thin plate splines interpolation. The registration method was developed in-house and demonstrated a registration accuracy of about the voxel size [Citation10]. However, the proposed method was not restricted to the use of this image registration method. Any DIR method could be used as long as the registration accuracy is acceptable.
The registration process resulted in one DVF for each control-CT. This DVF described the deformation between the planning-CT and a control-CT scan. Our registration method required a mean calculation time of (2.6 ± 1.5) minutes per fraction. The resulting transformation was used to transform the VOIs from the planning-CT scan to the control- CT for its visual examination. All transformed VOIs were reviewed by a radiation oncologist, thus evident registration errors could be excluded in this analysis. This exclusion was necessary in 19 of 327 CT scans (5.8%) distributed along five of 11 patients.
Deduction of an optimised 3D-correction vector from the DVF
Geometrical constraints for the optimisation process
Despite the deformations, both target volumes should be fully covered in each treatment fraction. Hence, at any time during the treatment, each voxel (volume element) of the tCTV/pCTV should be positioned within the tPTV/pPTV margin. Additionally, all voxels of the spinal cord should be positioned within the PRV for optimal dose sparing.
To calculate the optimised correction vector, an optimisation process was performed. This process considered pre-defined geometrical constraints of all VOIs that were considered relevant for patient alignment by the physician. In this proof-of-principle study, only one constraint C was defined for each VOI. To ensure target dose coverage and optimal OAR sparing, the constraint must be defined such that the transformation of each voxel of this VOI results in a position within a respective margin- extended VOI (). In this study, the CTV- to-PTV margin amounted for 3 mm (5 mm) for the tCTV (pCTV). To account for technical uncertainties, we restricted the constraint (margin extension) CtCTV in our optimisation function to 2 mm and the constraint CpCTV to 4 mm. Besides the target volumes, optimal re-positioning of the cord was also considered in the optimisation process. The PRV of the cord was constructed by adding a 5 mm margin to the cord. Therefore, analogous to the previous constraints, the constraint CSC for the spinal cord was defined as 4 mm. In this study, we refer to the margin-extended volumes as fair territories.
Figure 1. Schematic illustration of the three fair territories = margin-extended volumes. Left panel: planning situation: Delineated are the tCTV (red), pCTV (pink), spinal cord (blue) and their respective fair territories in shades of grey. Right panel: Same VOIs re-contoured on the changed anatomy prior to the optimised correction. Hatched areas indicate the volume fractions which are minimised in the optimisation process. Green arrows: Allowed voxel motion that does not violate the constraint for optimisation. Red arrows: Voxel motion that violates the constraint.
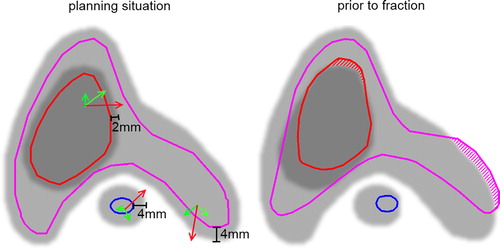
The constraints defined for the three VOIs were the same for all of the 11 patients. Each voxel of the three VOIs considered in the optimisation process must fulfil the defined constraints in each treatment fraction.
The optimisation process converged towards a rigid transformation, which maps the re-contoured VOIs inside the respective fair territories. The sum of all fraction volumes outside the fair territories was used as objective function. The minimum of the objective function is determined by a simplex algorithm [Citation11]. The proposed approach could be interpreted as a best fit of a rigid-body transformation to a given DVF, which described the deformations of the anatomy. The optimised vector resulting from this optimisation process included three translational and three rotational parameters.
Reference method: standard IGRT correction
To compare our method, a widely used standard (state-of-the-art) IGRT correction was simulated. Therefore, a rigid registration method considering translations and rotations was used to determine an IGRT correction vector. The registration method was based on mutual information [Citation12]; the bounding box encompassed the pCTV. The applied rigid registration method is included in our in-house developed treatment planning system [Citation13] and is regularly applied for image-guided positioning corrections in IMRT treatments for about 10 years. The resulting alignment quality is also subject to regular quality assurance performance tests.
Decision-making aid for re-planning
In the presence of extensive deformations it can be expected that the transformation determined in the optimisation process results in unacceptably large volume fractions located outside the fair territories. In these cases deformations cannot be compensated by a simple couch shift that maps the current, deformed anatomy as best as possible to the planning anatomy. Re-planning is required to handle these large deformations. Here, the proposed method can be used to introduce an action level for re-planning. In this study, we chose the action level to be 2 volume percent of each VOI. This means that a maximum 2% of each VOI outside the margin-extended volume was accepted. If this criterion was exceeded by any VOI, re-planning was assumed to be necessary in this study.
Results
The volume fractions located outside the margin-extended volumes were displayed for the spinal cord, the tCTV and the pCTV for one exemplary patient (patient 10) in . While the upper panel (no IGRT correction) showed an acceptable mapping of all three VOIs in only seven of 30 fractions, the two lower panels (IGRT) showed an increased number of fractions with acceptable VOI positioning: standard IGRT showed 13 acceptable positioning corrections and our proposed optimised IGRT showed even 25 of 30 acceptable corrections (in non-IGRT scenarios a further margin extension would typically be applied to correct for this worse alignment).
Figure 2. Residual volume fractions along the treatment course. Presented for one patient (patient 10) are the volume fractions outside the margin-extended volume (fair territory) of the spinal cord (blue), tCTV (red) and pCTV (pink) over a treatment course. The three panels show the outlying volume fractions, above: using no IGRT but patient positioning based on external markers; middle: using a standard IGRT correction determined from a rigid image registration including rotations; below: using the optimised IGRT correction determined from a vector field, including rotations.
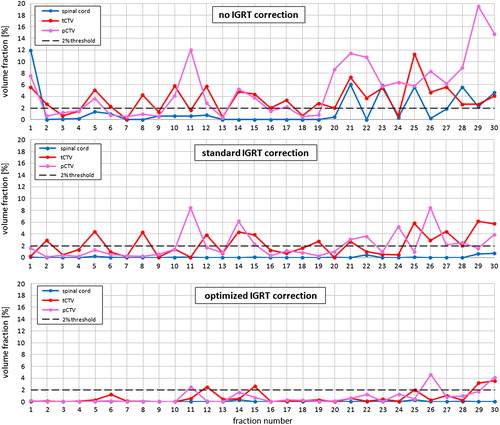
Comparison of the two IGRT strategies revealed that the optimised IGRT strategy is superior. The number of fractions with any of the volumes exceeding the 2% threshold was reduced considerably in this exemplary patient: from 17 to five of 30. Even though the registration box was fitted to the pCTV in the standard IGRT scenario, more fractions with a worse alignment of this specific VOI were observed in comparison to the proposed method: with eight versus three unacceptable positioning corrections for the pCTV.
Considering the whole patient cohort and different strategies, the increasing values in illustrated that the optimised IGRT method (with the defined constraints) was able to determine a rigid correction vector more frequently compared to the alternatives, despite the high flexibility of the anatomy in the head-and-neck region. In total 327 control-CT scans of 11 HNC patients were analysed. In 171 fractions ( = 52.3% vs. 22.6% in the standard IGRT strategy) the proposed method could determine an optimised vector meeting all defined constraints. These fractions were distributed across all patients.
Table I. Percent (number) of fractions resulting in a mapping of the VOIs with less than 2% of each VOI volume being located outside the margin-extended volume (fair territory). The values are presented for four different correction strategies.
Comparison of the standard IGRT strategy with a version of the optimised IGRT strategy that did not considered rotations in the optimisation process showed that, in more fractions, an acceptable alignment of the three VOIs was achieved with the latter strategy in all but one patient (# 9). This was also true, when comparing the standard IGRT strategy with the optimised IGRT correction strategy allowing for translational and rotational corrections.
The optimisation process to generate the optimised 3D vector took on average (1.5 ± 0.2) minutes in our cohort for the optimisation including rotational degrees of freedom.
Discussion
In this proof-of-principle study we present a new field of application for DVFs: The deformation described by the DVFs is used to determine an optimised treatment couch correction for the patient setup. This determination additionally allows introducing a threshold as a decision-making aid for re-planning.
Comparison of the different approaches for determination of a couch vector showed that the optimised IGRT strategy is superior in respect to a widely used method that uses rigid registration. This is expected because the criteria of the optimisation process focus on the proper mapping of all voxels of the relevant VOIs, independent of their image intensities. This approach can be understood as an “inverse” alignment. In contrast, using a “forward” alignment via rigid registration, the user cannot restrict the desired position of the VOIs after registration, because the image intensities dictate the mapping. The user can only choose the region for registration without knowing its impact of the weighting of the included content on the registration result. However, it is known that the size and site of the region used for registration influences the couch vector and thus the alignment of different head and neck structures [Citation6]. In our method, there is no need to think ahead regarding how the chosen region for registration might influence the alignment of the VOI, since no registration box definition is required. Due to the optimal VOI alignment in our method, the probability to find a vector for a rigid correction is higher. This means our method reduces the need for plan modification, since it is able to find satisfactory rigid correction parameters in more cases than the standard approach.
Furthermore, the proposed method can be used as a decision-making aid, which is highly desirable in the workflow of adaptive radiotherapy. Automation can help to standardise the re-planning process by making such decisions reproducible. Re-planning is necessary in the presence of large deformations, since no simple correction method can result in an acceptable mapping of the VOIs. A current study of Paganelli et al. proposes a decision-making index for re-planning based on automatic feature detection and its cohort-based ratio comparing the feature distances after rigid transformation and deformation analysis, derived by a DIR [Citation14]. If our method is used as a decision-making aid, more detailed information about the specific VOI violating the constraints can be achieved as well as the extent of this violation. For our method it is necessary to define an action level for re-planning. In this proof-of-principle study we used a threshold of 2% volume fraction. In all of the analysed patients, re-planning would have been necessary if this criterion was applied. It should be kept in mind that this criterion is a very strict one. Yet, the goal of our study was to demonstrate the potential of the proposed method only and not to present a statistical evaluation of the necessity to re-plan HNC patients. Therefore, further investigations with large patient cohorts are necessary to determine the appropriate threshold. If re-planning is performed during the treatment course, the method can also be used to choose the plan which is more suitable in subsequent fractions, by checking the re-planning criteria for both plans. This corresponds to a best-plan-of-the-day concept, since it is possible to choose the better option between the initial strategy and the re-planned one in subsequent fractions. Here, the better plan corresponds to a VOI alignment with smaller volume fractions outside the fair territories.
The idea of selecting patients with large deformations that might benefit from a plan adaptation has already been raised by van Kranen et al. [Citation7]. In their study, couch shifts were optimised based on rigid multiple region-of-interest registrations, instead of using only one box for registration. This approach requires the definition of multiple registration boxes and is limited to bony anatomy. The influence of the registration of single boxes on the actual alignment of the important VOIs, e.g. target volumes, needs to be considered by the physician in this approach. In our method the knowledge of the deformation is not limited to bony anatomy because a complete vector field is used.
In this proof-of-principle study, the proposed method was demonstrated using a DIR that was developed in-house [Citation10]. It is not important which specific DIR method is used. Previous studies have already shown the benefit of DIR applications in image-guided radiotherapy [Citation15]. Yet, if a DIR method is applied in daily routine, as a matter of course, only registration methods that have shown sufficiently high registration accuracy should be used. Nowadays, an unquestioned method to validate DIR results is still missing. Especially, the inter-observer variations of chosen references and its assessment for accuracy quantification represent a limiting factor [Citation16]. In the present study the uncertainty due to inter-observer variations could not be assessed and the visual inspection served as threshold to accept a specific individual DIR result. However, the majority of studies including DIR steps indicate that the resulting structure alignment is better than applying solely rigid registration methods, at least when accompanied by visual control [Citation17]. When using our method for IGRT correction or as criterion for re-planning, visual inspection of the re-contoured VOIs can be realised prior to treatment. This way the risk of gross errors due to mis-registrations can be minimised. An additional control instrument is the visual inspection of the rigidly transformed control-CT in relation to the planning-CT and its initially defined VOIs.
To demonstrate the method, geometrical constraints needed to be defined. In this study we defined the same constraints for the three VOIs in all patients. However, in daily routine, the constraints can be fixed individually for each patient at the request of the physician, e.g. dependent on the applied PTV margins. It is also possible to define several constraints for one VOI. For example, different constraints for the anterior-posterior and left-right direction could be used for the spinal cord. Currently, the optimisation process is limited to geometrical constraints only, in order to simplify matters. A further advancement of our method is to upgrade the geometrical constraints to dosimetric constraints, e.g. by using isodose lines instead of fixed margin-extensions. This could help to maintain the prescribed dose in OARs, since, e.g. the actual isodose lines do not necessarily correspond to the PRV contours. The need to consider dosimetric consequences for the CTV was previously indicated in a simplified approach [Citation18]. In our method, not only the constraints, but also the number and sites of the VOIs considered in the optimisation process can be chosen at will. Another step in a further development of the method can be the introduction of VOI-specific weighting factors. So far we have not considered a prioritisation of a single VOI.
Our method allows selecting the fractions that require re-planning. This is beneficial in comparison to an unselective (daily) online re-planning strategy because it reduces the treatment time, which is an important aspect in clinical practice. To date, this proposed optimisation approach is with 1.5 minutes much faster than the published times for daily online re- planning processes, which are reported to take at least 5 minutes [Citation19]. In the current proof-of-principle implementation, parallelisation and optimisation of the used algorithms are not exploited. Thus, there is the potential to further speed-up our method. A possible workflow integrating our method for the re-planning process is to first check if an optimised vector can be determined. If not, re-planning is initialised. This scenario takes 6.5 minutes, assuming the above-mentioned time specifications. However, if an optimised vector can be determined, only 1.5 instead of 5 minutes is required. In our study in more than half of all fractions re-planning was not necessary despite the strict constraints. Less strict constraints would further increase the number of fractions that do not require re-planning, and thus save further treatment time.
In summary, the proposed method has two main advantages: First, the correction parameters determined by this method ensure controlled mapping of the VOIs despite small deformations. Our method does not require manually choosing a region for registration when using IGRT. Second, the proposed method can also serve as a decision-making aid for re-planning. Thus, our method helps to lower the efforts of more complex correction strategies, such as unselective daily online re-planning. This means the method can help to standardise the re-planning process and make re-planning decisions more reproducible, which is highly desirable in the workflow of adaptive radiotherapy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
EMS was supported by the postdoc fellowship program of the University Hospital Heidelberg.
References
- Van der Put RW, Kerkhof EM, Raaymakers BW, Jürgenliemk-Schulz IM, Lagendijk JJ. Contour propagation in MRI-guided radiotherapy treatment of cervical cancer: The accuracy of rigid, non-rigid and semi-automatic registrations. Phys Med Biol 2009;54:7135–50.
- Peroni M, Ciardo D, Spadea MF, Riboldi M, Comi S, Alterio D, et al. Automated segmentation and online virtual CT in head-and-neck adaptive radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:e427–33.
- Gaede S, Olsthoorn J, Louie AV, Palma D, Yu E, Yaremko B, et al. An evaluation of an automated 4D-CT contour propagation tool to define an internal gross tumour volume for lung cancer radiotherapy. Radiother Oncol 2011;101:322–8.
- Thor M, Petersen JB, Bentzen L, Hoyer M, Muren LP. Deformable image registration for contour propagation from CT to cone-beam CT scans in radiotherapy of prostate cancer. Acta Oncol 2011;50:918–25.
- Lavoie C, Higgins J, Bissonnette J-P, Le LW, Sun A, Brade A, et al. Volumetric image guidance using carina vs spine as registration landmarks for conventionally fractionated lung radiotherapy. Int J Radiat Oncol Biol Phys 2012;84:1086–92.
- Stoiber EM, Schwarz M, Huber PE, Debus J, Bendl R, Giske K. Comparison of two IGRT correction strategies in postoperative head-and-neck IMRT patients. Acta Oncol 2013;52:183–6.
- Van Kranen S, van Beek S, Mencarelli A, Rasch C, van Herk M, Sonke JJ. Correction strategies to manage deformations in head-and-neck radiotherapy. Radiother Oncol 2010;94:199–205.
- Giske K, Stoiber EM, Schwarz M, Stoll A, Muenter MW, Timke C, et al. Local setup errors in image-guided radiotherapy for head and neck cancer patients immobilized with a custom-made device. Int J Radiat Oncol Biol Phys 2011;80:582–9.
- Chen AM, Farwell DG, Luu Q, Donald PJ, Perks J, Purdy JA. Evaluation of the planning target volume in the treatment of head-and-neck cancer with intensity-modulated radiotherapy: What is the appropriate expansion margin in the setting of daily image-guidance?. Int J Radiat Oncol Biol Phys 2011;81:943–9.
- Malsch U, Thieke C, Huber PE, Bendl R. An enhanced block matching algorithm for fast elastic registration in adaptive radiotherapy. Phys Med Biol 2006;51:4789–806.
- Nelder JA, Mead R. A simplex method for function minimization. Comput J 1965;7:308–13.
- Maes F, Collington A, Vandeermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 1997; 16:187–98.
- Bendl R, Hoess A, Schlegel W. Virtual simulation in radiotherapy planning. Lect Notes Comput Sci 1995;905: 287–92.
- Paganelli C, Peroni M, Riboldi M, Sharp GC, Ciardo D, Alterio D, et al. Scale invariant feature transform in adaptive radiation therapy; a tool for deformable image registration assessment and re-planning indication. Phys Med Biol 2013;58:287–99.
- Elstrom UV, Wysocka BA, Muren LP, Petersen JB, Grau C. Daily kv cone-beam CT and deformable image registration as a method for studying dosimetric consequences of anatomic changes in adaptive IMRT of head and neck cancer. Acta Oncol 2010;49:1101–8.
- Mencarelli A, van Beek S, van Kranen S, Rasch C, van Herk M, Sonke JJ. Validation of deformable registration in head and neck cancer using analysis of variance. Med Phys 2012;39:6879–84.
- Hardcastle N, Tome WA, Cannon DM, Brouwerw CL, Wittendorp PW, Dogan N, et alA multi-institution evaluation of deformable image registration algorithms for automatic organ delineation in adaptive head and neck radiotherapy. Radiat Oncol 2012;7:90.
- Yue NJ, Kim S, Lewis BE, Jabbour S, Narra V, Goyal S, et al. Optimization of couch translational corrections to compensate for rotational and deformable target deviations in image guided radiotherapy. Med Phys 2008;35:4375–85.
- Ahunbay EE, Peng C, Godley A, Schultz C, Li XA. An on-line replanning method for head and neck adaptive radiotherapy. Med Phys 2009;36:4776–90.
