Abstract
Background. Premenopausal patients treated with adjuvant chemotherapy often develop early menopause and thus, may encounter significant bone loss. We studied the long-term effects of chemotherapy-induced ovarian dysfunction on bone mineral density in breast cancer patients. Material and methods. The effect of menstrual status after adjuvant chemotherapy on bone mineral density (BMD) was examined in 29 premenopausal breast cancer patients. Results. During 10 years of follow-up, nearly 90% of women developed menstrual irregularities or amenorrhea. The total bone loss at the lumbar spine was −5.4% in women with preserved menstruation, −15.3% in those with irregular menses and −13.2% in amenorrheic women 10 years after adjuvant chemotherapy. The changes in lumbar spine BMD correlated significantly with menstrual function. Both amenorrhea and menstrual irregularities shortly after chemotherapy increased the risk of osteoporosis later on: one third of women with ovarian dysfunction developed osteoporosis of the lumbar spine during the follow-up. Osteopenia before adjuvant therapy predicted an increased risk for osteoporosis. Conclusion. The present study with a unique follow-up period of 10 years shows that ovarian dysfunction leads to long-term deleterious BMD changes and predisposes breast cancer survivors to osteoporosis.
Breast cancer survivors are at an increased risk for osteoporosis and fracture compared with women in general [Citation1,Citation2]. In the observational component of the Women Health Initiative Study (WHI), the rate of fractures was increased by 15% in breast cancer survivors compared with women with no cancer history. Clinical vertebral fractures were significantly more common among survivors who had a breast cancer diagnosis before 55 years of age, probably reflecting the effect of chemotherapy-induced menopause on bone loss [Citation2].
Premenopausal breast cancer patients receiving adjuvant chemotherapy have a high risk of developing hypogonadism. Combination chemotherapy with cyclophoshamide, methotrexate and fluorouracil (CMF) or fluorouracil, doxorubicin and cyclophoshamide (FAC) causes premature menopause in 63–96% of premenopausal women within one year, with older patients and those receiving higher cumulative doses of cyclophosphamide having the highest risk [Citation3,Citation4]. Estrogen balances osteoblast and osteoclast activity in premenopausal women [Citation5]. Estrogen deficiency due to menopause in turn results in an imbalance of bone remodeling and leads to bone loss. Previous studies have demonstrated that up to 7–8% of lumbar spine bone mass is lost during the first year after chemotherapy-induced early menopause [Citation6,Citation7].
We have previously reported that the bone loss rate is most rapid during the first few years after chemotherapy-induced menopause and although the bone loss slows down thereafter, it is still marked five years after adjuvant therapy [Citation8]. Here we present the extended 10-year follow-up results of the effect of chemotherapy-induced hypogonadism on bone mineral density (BMD) in premenopausal breast cancer patients.
Material and methods
Material
The study population consisted of 141 premenopausal women with newly diagnosed node-positive breast cancer who participated in adjuvant clodronate (Bonefos®, Bayer Schering Pharma) trial between 1990 and 1993 at the Department of Oncology, Helsinki University Hospital, in Helsinki, Finland. Exclusion criteria were the following: 1) Karnofsky performance index below 70%; 2) other malignancies; 3) peptic ulcer; 4) serum creatinine over 150 umol/l; and 5) pregnancy. Premenopausal status at entry was defined as regular menstruation or last menstrual cycle within three months and serum follicle stimulating hormone (FSH) levels less than 30 U/l.
After surgery with mastectomy or breast- conserving resection and axillary evacuation, all patients had postoperative radiotherapy (50 Gy in 25 fractions) to regional lymph nodes and to operative scar or remaining breast. In addition, women received six cycles of CMF chemotherapy consisting of 600 mg/m² cyclophosphamide, 40 mg/m² methotrexate and 600 mg/m² fluorouracil administered intravenously at three-week intervals. The patients were also randomly assigned to either 1600 mg oral clodronate daily for three years (clodronate group) or no further therapy (control group).
We have previously reported the effects of clodronate on BMD changes after adjuvant chemotherapy [Citation6,Citation8,Citation9]. The current study comprised only premenopausal patients in the control group and those having received clodronate (n = 63) were excluded. Data from a further 49 patients were excluded because of breast cancer death (n = 29) or recurrence (n = 6), discontinued follow-up (n = 1), medications or diseases possibly affecting bone metabolism (n = 7), inadequate adjuvant therapy (n = 1), pregnancy (n = 1) or unavailable information on menstrual function (n = 4). Overall, 29 metastasis-free patients were eligible for analyses at 10 years of follow-up.
One year after adjuvant chemotherapy the patients were divided into three groups according to menstrual status: regular ( = monthly) menses, irregular menses and amenorrhea. Irregular menses were defined as last menstrual cycle within one to six months and amenorrhea as absent menstruation for at least six months.
Methods
Informed consent was obtained from all participants. The local ethics committee approved the study protocol. Staging investigations included clinical investigation, thoracal x-ray, liver ultrasound, and bone scintigraphy. Basic laboratory tests consisted of complete blood count and sedimentation rate, liver enzymes, serum creatinine and electrolytes. Patient interviews, bone scintigraphy and measurements of FSH, luteinizing hormone (LH) and estradiol were performed before chemotherapy and at one, two, three, five and 10 years thereafter. Clinical investigation and basic laboratory safety tests were repeated with a radiologic examination, if necessary, according to the usual follow-up practice.
BMD (grams per square centimeter) was measured by dual-energy, x-ray absorptiometry using a Hologic QDR-1000 densitometer (Hologic Inc, Waltham, MA, USA). BMD was measured at the lumbar vertebrae (L1–4) and at the femoral neck, femoral trochanter, intertrochanteric and total femoral area in the right femoral area before therapy and at one, two, three, five and 10 years thereafter.
According to the World Health Organization (WHO) criteria, BMD T scores −2.5 SD or more below normal peak bone mass were classified as osteoporotic, T-scores between −1 and −2.5 SD were considered osteopenic and those over −1 SD normal [Citation10].
Statistical methods
The effect of menstrual status after chemotherapy on BMD was tested by repeated-measures analysis of variance model with menstrual status as grouping variable and BMD changes as dependent variable. Other comparisons between groups were performed by t-test. Four patients (three patients in the irregular menses group and one patient in the amenorrhea group) received bisphosphonate therapy because of osteoporosis after the first five years of follow-up and the BMD values were kept unchanged thereafter. All patients were included in the 10-year analyses of BMD changes.
Results
Effect of chemotherapy on BMD
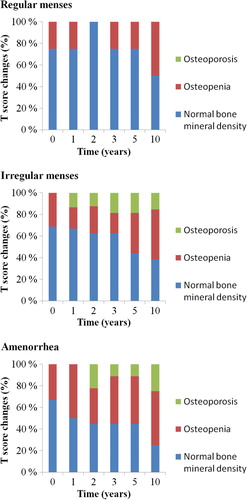
Women in the regular menses group were significantly younger than those in the irregular menses (p = 0.002) or amenorrhea groups (0.004) – otherwise the groups were well balanced according to the pretreatment characteristics (). One year after chemotherapy four patients had regular menses and 16 had irregular menses. Nine patients had developed amenorrhea at an average age of 48 years. The four patients with regular menses one year after chemotherapy still continued to menstruate at five years. Seventy-five percent of women (12 of 16 patients) with irregular menses at one year, however, developed amenorrhea during five years of follow-up at an average age of 48 years. The total bone loss at lumbar spine after 10 years was significantly greater in patients with chemotherapy-induced ovarian dysfunction than in those who preserved menstruation ().
Figure 1. T-score (A) and percentual (B) BMD changes in the lumbar spine according to menstrual status.
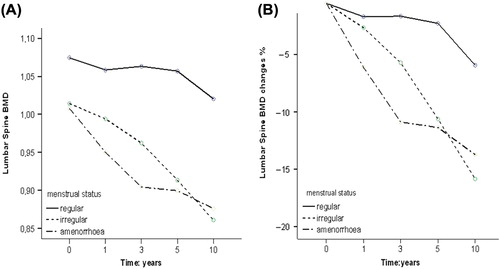
Table I. Pretreatment characteristics of the patients according to menstrual status.
The changes in lumbar spine BMD during 10 years since chemotherapy correlated significantly with menstrual function (p = 0.015). No correlation was found between femoral neck BMD and menstrual function (p = 0.765).
Effect of menstrual function on osteoporosis-free survival
None of the patients had osteoporotic BMD values before adjuvant therapy. Nine patients had osteopenia of the lumbar spine, eight patients at the femoral neck and three patients at the total femoral area already at baseline ().
During the 10 years of follow-up, eight of 29 patients developed osteoporosis in the lumbar spine. None of the four women with regular menses was diagnosed with lumbar spine osteoporosis. Five of 16 women in the irregular menses group and three of nine women in the amenorrhea group developed osteoporosis of the lumbar spine. Thus, 10-year spinal osteoporosis-free survival (OPFS) was 100% in the regularly menstruating patients, 69% in those with irregular menses and 67% in amenorrheic patients. Only one patient was diagnosed with femoral neck osteoporosis – this 28-year-old woman in the regular menses group had osteopenic T-scores already at baseline.
Women with pretreatment osteopenia were at an increased risk of osteoporosis. Six of nine (67%) patients who developed osteoporosis at any site had osteopenia of the lumbar spine at baseline. Only two of 20 (10%) women with normal pretreatment BMD at lumbar spine were diagnosed with osteoporosis during the 10 years of follow-up. Ten-year T-score changes are presented in .
Discussion
We have previously reported that women who undergo premature menopause after adjuvant chemotherapy experience a period of accelerated bone loss [Citation6,Citation8]. The present longitudinal study with a unique follow-up period of 10 years shows that ovarian dysfunction leads to long-term deleterious BMD effects and predisposes breast cancer survivors to osteoporosis. Patients with ovarian dysfunction lost nearly three-fold more of their lumbar spine BMD as compared to those with preserved menstruation. In line with the BMD findings, women with ovarian dysfunction shortly after chemotherapy were at an increased risk of spinal osteoporosis.
Corresponding to previous literature, most women over 40 years of age developed menstrual irregularities or amenorrhea after chemotherapy. Seventy-five percent of women with irregular menses shortly after chemotherapy, encountered menopause within the next few years. The total amount of bone lost during 10 years of follow-up did not differ between those with early (within one year) or late (within five years) onset of amenorrhea – the risk of osteoporosis was identical as well. Thus, menstrual irregularities shortly after chemotherapy seemed to carry a similar risk of osteoporosis as did an early onset of amenorrhea.
As awaited, the risk of osteoporosis was associated with low baseline BMD levels. Nearly 70% of the patients who developed osteoporosis during the 10 years of follow-up had osteopenia at baseline. This is in agreement with results of the large aromatase inhibitor studies in postmenopausal women. In the ATAC trial (Arimidex and Tamoxifen, alone or in combination) comparing anastrotzole and tamoxifen as adjuvant therapy in postmenopausal breast cancer, no woman with a normal BMD at baseline became osteoporotic during five years of follow-up and only those patients with osteopenia were at risk of developing osteoporosis [Citation11]. Equally, normal BMD before aromatase inhibitor therapy offered protection against osteoporosis in the IES (The Intergroup Exemestane) and MA.17 trials, with the former studying exemestane and the latter letrozole after tamoxifen therapy [Citation12,Citation13].
The American Society of Clinical Oncology (ASCO) recommends BMD screening and bone health promoting lifestyle advice including calcium and vitamin D substitution for all premenopausal breast cancer patients with therapy-associated premature menopause. BMD monitoring is encouraged for women with baseline osteopenia or other risk factors [Citation14]. According to the present findings, BMD screening might be recommended for premenopausal women with adjuvant therapy-induced amenorrhea and also for those with menstrual irregularities.
To our knowledge, the current study is the longest prospective follow-up report of the effects of adjuvant chemotherapy on BMD of premenopausal breast cancer patients. The number of patients included is relatively small, however, and we do not have data on the occurrence of osteoporotic fractures, which are the most important limitations of the present study. Our results are, however, supported by several previous reports [Citation6–8].
Conclusion
In summary, ovarian dysfunction after adjuvant chemotherapy leads to long-term detrimental BMD effects and predisposes breast cancer survivors to osteoporosis. A great majority of women experience ovarian dysfunction and those who do, have significant bone loss and an increased risk of osteoporosis 10 years after adjuvant therapy. Both amenorrhea and menstrual irregularities shortly after chemotherapy increase the risk of osteoporosis later on. Osteopenia before adjuvant therapy predicts an increased risk for osteoporosis. BMD screening in recommended for all premenopausal women with therapy-associated ovarian dysfunction.
Declaration of interest: Leena Vehmanen: Scientific meeting participation. Tiina Saarto has received research funding from GlaxoSmithKline for another project. The remaining authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kanis JA, McCloskey EV, Powles T, Paterson AHG, Ashley S, Spector T. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer 1999;79:179–81.
- Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, et al. Fracture risk among breast cancer survivors: Results from the Women's Health Initiative Observational Study. Arch Intern Med 2005;165:552–8.
- Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: Pathogenesis and management. J Clin Oncol 2000;18: 1570–93.
- Ramaswamy B, Shapiro CL. Osteopenia and osteoporosis in women with breast cancer. Semin Oncol 2003;30:763–75.
- Oursler MJ, Landers JP, Riggs BL, Spelsberg TC. Oestrogen effects on osteoblasts and osteoclasts. Ann Med 1993;25: 361–71.
- Saarto T, Blomqvist C, Välimäki M, Mäkelä P, Sarna S, Elomaa I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: A randomized study in premenopausal breast cancer patients. J Clin Oncol 1997;15:1341–7.
- Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol 2001; 19:3306–11.
- Vehmanen L, Saarto T, Elomaa I, Mäkelä P, Välimäki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment.Eur J Cancer 2001;37:2373–8.
- Saarto T, Vehmanen L, Blomqvist C, Elomaa I. Ten-year follow-up of 3 years of oral adjuvant clodronate therapy shows significant prevention of osteoporosis in early-stage breast cancer. J Clin Oncol 2008;26:4289–95.
- WHO Study Group. Assessment of fracture risk and its application to postmenopausal osteoporosis. World Health Organization Technical Series 843, 1984.
- Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial. J Clin Oncol 2008;26:1051–7.
- Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, et al. Intergroup Exemestane Study Group. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): A randomised controlled study. Lancet Oncol 2007;8:89–91.
- Perez E, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: A companion study to NCIC CTG MA.17. J Clin Oncol 2006;24:3629–35.
- Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003;21:40–2.