Abstract
Background. Alopecia is a frequently occurring side effect of chemotherapy that often can be prevented by cooling the scalp during the infusion. This study compared effects and costs of scalp cooling with usual general oncological care, i.e. purchasing a wig or head cover. Material and methods. Scalp-cooled patients (n = 160) were compared with non-scalp-cooled patients (n = 86) at 15 Dutch hospitals. Patients were enrolled prior to anthracycline and/or taxane-based chemotherapy for several types of cancer between 2007 and 2008. Cost-effectiveness of scalp cooling compared with that of usual care was determined by the ratio of costs to quality adjusted life years (QALYs). Costs for scalp cooling (machines and nursing time), hair dressers, wigs and head covers were estimated from a societal perspective. QALYs were measured using the Short Form-36. Results. Scalp cooling reduced the use of a wig or head cover by 40%, but wigs were still purchased unnecessarily by 38% of scalp-cooled patients. Average societal costs decreased therefore only by €269 per patient due to scalp cooling (p = 0.02). Given the eligibility for scalp cooling at the time, the insignificant difference in QALYs resulted from a balance of the benefits for those patients with successful scalp cooling and those without success. For the Dutch, given the generally accepted threshold of willingness to pay for a QALY (between €20 000 and €40 000), scalp cooling was cost-effective, therefore justifying the choice of scalp cooling or purchasing a wig or head cover. Conclusion. Given the right indication, cost-effectiveness might be improved further by postponing wig and head cover purchases, by improving scalp cooling efficacy, as well as using the scalp cooling capacity more intensively.
Chemotherapy frequently induces alopecia, making cancer visible to the outside world. It is often considered an inevitable side effect of chemotherapy, a temporary burden that should be taken for granted. It has, however, a negative impact on the wellbeing of many cancer patients [Citation1–3], which – according to some – has been underestimated or ignored by both oncology nurses and medical doctors [Citation4,Citation5].
During the last five years prevention of chemotherapy-induced alopecia (CIA) has become a topic of supportive care. Of the ± 91 000 patients newly diagnosed with cancer in the Netherlands in 2009, 27% received chemotherapy as part of their primary treatment (source: Netherlands Cancer Registry, unpublished data). From 2000 to 2008, the proportion of breast cancer patients who received chemotherapy as part of initial treatment increased by 40% [Citation6]; the proportion even doubled for patients with gastro-intestinal and lung cancer (source: Eindhoven Cancer Registry, unpublished data). The administration of chemotherapy is still increasing. Although the incidence of severe CIA is generally lower in the case of targeted therapies and oral chemotherapy, these agents are often combined with regular cytotoxic drugs that do cause CIA.
Scalp cooling is the most effective method to prevent CIA. In currently used chemotherapies scalp cooling equipment prevents severe hair loss in about half of the patients [Citation7–9]. Prevention of CIA by pharmaceutical agents is not very promising as a clinical application in the near future [Citation10–12], neither are new non-pharmaceutical methods such as electrogenesis or laser therapy [Citation13,Citation14]. The use of scalp cooling has increased worldwide and CIA does not seem to be inevitable anymore.
Medical oncologists have to choose whether they want to offer scalp cooling as a service to patients at risk of severe CIA. Effectiveness and safety, but also costs and attribution of costs play a role in this decision. As part of an introduction program for scalp cooling we therefore compared costs and effects of scalp cooling with those of usual care, i.e. in the Netherlands the choice of purchasing a wig or head cover, when cancer patients are faced with CIA.
Material and methods
Patients and setting
In this non-randomised prospective study scalp-cooled patients (n = 160) were compared with non-scalp-cooled patients with the same chemotherapy regimens (n = 86). While the effectiveness of scalp cooling has been proven, also in trials [Citation7,Citation8], it would be unethical to randomise patients. Patients were eligible if they received a chemotherapy schedule with the potential of inducing severe CIA and therefore scalp cooling was commonly applied. Patient characteristics were well balanced except for the proportion of patients receiving 5-fluorouracil, epirubicine and cyclophosphamide (FEC) (). From January 2007 to December 2008, patients were included from 15 hospitals, two of which did not offer scalp cooling. Patients in the scalp cooling hospitals who did not choose scalp cooling could participate in the non-scalp-cooled group.
Table I. Socio-demographic and clinical characteristics of patients treated with or without scalp cooling (n = 246).
Scalp cooling was performed using the Paxman system (type PSC1 or PSC2) with a standardised cooling time: from 30 minutes before the chemotherapy infusion to 90 minutes after stopping the infusion.
Approval for this study was obtained from the Medical Ethics Committees and all participating patients signed forms of informed consent.
Measures
Patients received four sets of questionnaires (see sections below) with return envelopes and were asked to complete them at home before the start of chemotherapy and three weeks, six and 12 months after completing chemotherapy. Patients were eligible for analysis if they completed at least the first and second questionnaire.
Costs
CIA-related costs were estimated from the start of chemotherapy until 12 months after completion. At that time, the hair has grown to such an extent, that the majority of patients are satisfied and stop wearing a wig or head cover [Citation15]. In order to estimate from the societal perspective, we took into account all health effects and changes in resource use caused by scalp cooling. Due to the short time line, costs were not discounted. Costs were converted to the 2010 price level, using the general Dutch consumer price index [Citation16].
Patients reported the cost of wigs and head covers from the start of chemotherapy until six months after completing chemotherapy. It was assumed that patients did not buy additional wigs or head covers after that time. Costs of hair dressers were estimated for all patients up to 12 months following chemotherapy. Other (health) care requirements (e.g. informal care) and productivity (e.g. return to work) were assumed to be unaffected by scalp cooling.
Hospital costs included time spent by nurses and equipment needed for scalp cooling. In each hospital a maximum of 10 oncology nurses (range 2–10) completed a questionnaire. Nurses reported the time required to provide information about CIA and the performance of scalp cooling, i.e. fitting and cleaning the cap. Nursing time was valued at gross wages [Citation17]. Equipment costs were collected for two years and included the machine, caps, coolant and maintenance costs. The economic lifetime of the machine and caps was assumed to be 10 years [Citation17]. Annual costs were divided by the annual number of sessions. The sessions were recorded by a data manager for all chemotherapy patients treated with scalp cooling in day care during the two-year study period, including use by non-study participants. Scalp cooling is currently not used during clinical chemotherapy treatment.
Additional space required for storage of the equipment was negligible. Also electricity, costs of cleaning the cap and use of disposable gauze bandages for hygienic application of the cool cap's chin strap were too minimal to take into account. No extra treatment chairs or beds were required for scalp cooling in the day care units.
Quality of life
Utilities represent the valuation of quality of life (QoL) of the patients, on a scale from zero (as bad as death) to one (perfect health). Quality Adjusted Life Years (QALY) takes into account both the quantity and quality of life generated by health care interventions.
Patients reported general health-related QoL using the Short Form-36 (SF-36). From the SF-36 we derived SF-6D scores which were used to calculate utilities [Citation18]. Together with the EQ-5D, HUI and QWB the SF-36 derived SF-6D is one of the methods used for economic evaluations from a societal perspective [Citation19,Citation20]. The utilities provide societal valuation and offer the possibility of comparison of the impact with other medical interventions.
For sensitivity analysis we also obtained valuations by the patients themselves using a Visual Analogue Scale (VAS), ranging from 0 (worst imaginable QoL) to 100 (perfect QoL). The VAS values were transformed to a utility scale, using the power transformation 1-(1-VAS/100)1,61 [Citation21]. QALYs were calculated from the area under the utility curves for the entire study period.
Statistics
Socio-demographic and clinical characteristics were compared between scalp-cooled and non-scalp-cooled patients using the χ2-test. Cost analyses were performed with Stata 9.2 (StataCorp, College Station, TX, USA). To reduce possible bias in these analyses due to missing data, multiple imputation by chained equations was used [Citation22], with 10 iterations for the switching regression model. For each missing utility or cost measure, an imputation regression model was used that included age, gender, chemotherapy type, number of chemotherapy sessions, setting (adjuvant or palliative), number of scalp cooling sessions, SF36 and VAS for QoL and cost measurements at all moments. Differences in QALYs or use of head covering and costs between scalp-cooled and non-scalp-cooled patients were analysed using the bootstrap method.
Base case cost-utility analysis was determined comparing societal costs from the start until 12 months after the stop of chemotherapy and QALYs based on the SF36. Sensitivity analyses were performed using VAS for QoL and costs of the equipment. Costs of equipment were halved, reflecting doubling the number of scalp-cooled patients who use the machine in a hospital.
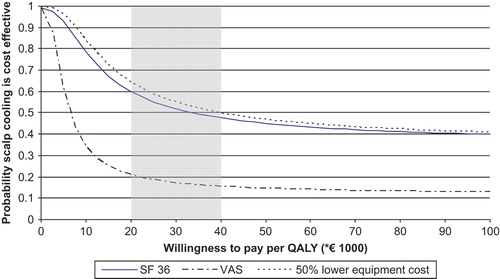
The societal Willingness To Pay (WTP) for a QALY is an indicator of cost-effectiveness, comparing WTP * QALYs gained versus costs. Then the probability that a strategy is cost-effective is graphed as a function of WTP in an acceptability curve [Citation23]. Cost-effectiveness is plausible when the probability that scalp cooling is effective (y-axis) exceeds 0.5. The Dutch economic threshold for WTP is assumed to be between €20 000 and €40 000 per QALY [Citation24,Citation25].
Results
Costs
The average societal costs decreased €269 (95% CI €46–€493; p = 0.02) per scalp-cooled patient compared to usual care ().
Table II. Mean cost per scalp-cooled and non-scalp-cooled patients from the start of chemotherapy until 12 months after completion of chemotherapy.
Patient's costs associated with wigs, other head covers and hair dressers were missing for respectively 5%, 5% and 10% of them. Non-scalp-cooled patients spent significantly more money (mean difference €534, 95% CI €314–€754; p < 0.001) on wigs and head covers and less on hair dressers (mean difference €82, 95% CI €46–€119; p < 0.001) from the start of chemotherapy to six months after its completion (). Overall, the mean price for a wig was €616 (range €43–€3000), €265 was the standard refund by health insurance companies in 2007 and 2008, being about 45% of wig costs. Purchasing a wig was reported by 52% of scalp-cooled patients versus 77% of non-scalp-cooled patients: health insurance companies saved refund for wigs in about 25% of the chemotherapy patients who were at risk of severe CIA. As several patients had more than one wig during the follow-up period, the mean costs per patient could be higher than the mean price for a wig, as is the case in the non-scalp-cooled group (mean costs per patient €946).
When addressing only patients who purchased a wig, scalp-cooled patients bought a mean number of 1.8 wigs versus 2.0 for non-scalp-cooled patients, mean prices per wig were respectively €541 and €653.
Eligibility for scalp cooling varied between hospitals regarding types of cancer and chemotherapy. Non-scalp-cooled patients represented 56% of those treated in the two hospitals that did not offer scalp cooling.
One hundred and eight nurses completed the questionnaire on time expenditure. Nurses spent a mean of 10 minutes per patient on information about scalp cooling, which amounted to €5 per patient. Mean nursing time was 36 minutes per patient (range 9–81) and was assessed at €18 per scalp cooling session. Time needed to plan the cooling sessions was negligible (mean 10 minutes per week, range 0–35 minutes).
Nurses performed 1862 scalp cooling sessions per year. The 13 hospitals used six two-person scalp cooling machines and 17 single-person machines. The mean costs of equipment amounted to €21 per cooling session, with a mean of 144 sessions per hospital per year. Patients underwent a mean of 4.2 scalp cooling sessions.
The mean costs of scalp cooling were €183 per patient per hospital, whereby €94 were equipment costs (machine including caps, coolant and maintenance) and €89 nursing costs.
Quality of life
For the four measurements in time, SF-6D scores were missing for respectively 6%, 4%, 19% and 26% of the patients. VAS scores were missing forrespectively 1%, 2%, 15% and 22% of the patients. According to the SF-6D and VAS, there was no significant difference in QALYs between scalp-cooled and non-scalp-cooled patients ().
Table III. Mean utility and quality adjusted life years (QALYs) in scalp-cooled and non-scalp-cooled patients.
Cost-effectiveness
The probability that scalp cooling was cost-effective compared to no scalp cooling depended on the WTP (). For low values of the maximum WTP for a QALY (up to €34 000), the probability of being cost-effective was in favour of scalp cooling (above 0.5). For higher values of the WTP this probability decreased and chemotherapy without scalp cooling became preferable (below 0.5). Since the turning point of cost-effectiveness was within the acceptable range of the WTP for a QALY in the Netherlands, both strategies are acceptable from the societal point of view.
Sensitivity analyses
When using VAS for QoL valuation, the difference between scalp-cooled and non-scalp-cooled patients was somewhat more pronounced in favour of non-scalp-cooled patients than for the SF-6D (). Therefore, the line of the acceptability curve decreases (), indicating that scalp cooling is less likely to be cost-effective.
When the number of patients using the scalp cooling equipment in a hospital is doubled, equipment costs would amount to €12 per session and mean costs for the hospital would become €136 per scalp-cooled patient. In this sensitivity analysis, the difference in societal costs became €316 for scalp-cooled versus non-scalp-cooled patients (95% CI €93–€540, p = 0.01). Then the turning point of the probability that scalp cooling is cost-effective (i.e. 0.5) is about €40 000 per QALY. That is the upper value of the Dutch economic threshold for WTP, indicating that scalp cooling could be considered cost-effective ().
Discussion
Given the indications for treatment with chemotherapy and scalp cooling at the time of this study, scalp cooling appeared to be less expensive than usual care, i.e. purchasing a wig or head cover. Societal savings were €269 per scalp-cooled patient, but there was no significant difference in QALYs compared to non-scalp-cooled patients. Using scalp cooling saved €452 for patients, but entailed €183 extra costs per patient for hospitals. All in all, it seems justified to offer both options to the patient.
Assuming 24 500 patients a year with chemotherapy as part of their primary treatment (source: Netherlands Cancer Registry), whereby half would be faced with severe CIA, and half of them would choose scalp cooling, then total savings based on about 6000 patients per year in the Netherlands would amount to €1 500 000.
To our knowledge, cost-effectiveness of scalp cooling has never been investigated. Only one Willingness To Pay study on CIA found that lung cancer patients were willing to pay €83 per three-weekly chemotherapy cycle to reduce the risk of CIA from 40% to 5% [Citation26]. The WTP depended on the perceived impact of CIA and was higher among females with a higher income.
Cost-effectiveness of scalp cooling can easily be improved by decreasing the costs of anticipated head cover purchase [Citation27]. Secondly, costs will be reduced when a higher proportion of patients is satisfied with the scalp cooling result and thus do not feel the need to wear head covering, which is now, e.g. about 50% among patients with FEC chemotherapy [Citation28]. Therefore, scalp cooling should not be offered to patients with chemotherapy schedules in which scalp cooling is ineffective, i.e. TAC chemotherapy [Citation28]. Furthermore, research should be performed to further improve the efficacy of scalp cooling, e.g. by optimising scalp cooling times and temperature. Third, savings on nursing staff might be obtained when nursing assistants or volunteers would be trained to apply scalp cooling.
Societal costs may also be reduced if the purchase of machines is aligned to the number of cooling sessions. A hospital that owns one machine can treat at least one patient a day, but in this study on average fewer than three patients a week underwent scalp cooling.
More intense usage of scalp cooling lowered the costs for the machine per session from €24 to €12, but will increase the costs of nursing time. However, while in one hospital nurses spent extra time due to additional methods of fitting the cap – which are not used in any other hospital – nursing time per patient has been somewhat overestimated in this study. The occupation of a bed or chair may become an important additional cost aspect for the cost-effectiveness of scalp cooling, whereas occupation grades of day care units rise. Consequently, cost limits for planning scalp cooling is important, e.g. by shortening the post-infusion cooling time [Citation29].
For patients who had purchased a wig, somewhat higher prices per wig were found in the non-scalp-cooled patient group, as also a somewhat higher mean number of wigs per patient. This might be explained by scalp-cooled patients who buy the wig as a precaution and therefore might spend somewhat less money, but also do not buy an additional wig when scalp cooling is successful.
Health-related QoL as measured with a generic questionnaire was comparable for scalp-cooled and non-scalp-cooled patients. The benefits for successful scalp-cooled patients were probably balanced by those without success [Citation1], which is 50% of those on FEC chemotherapy. Results can be improved when optimal temperatures and cooling times per chemotherapy type are known.
We used the SF-36 derived SF-6D questionnaire as we expected it to be more sensitive in measuring the effects of scalp cooling than for example the EQ-5D, although no Dutch tariff is available for this questionnaire. We do not expect that using the EQ-5D would have changed our results. On the one hand, the EQ-5D does result in lower valuations than the SF-6D [Citation30], but on the other hand, Dutch valuations are higher than UK valuations [Citation31], which may offset each other. Furthermore, as both the SF-6D valuations and the VAS results did not differ between both groups, it is not to be expected that the results would had been different when using the EQ-5D or other generic QoL measures.
This non-randomised study has some limitations. First the approach to the supply of wigs and head covering differs between countries. Therefore, our cost-effectiveness model may have to be adapted according to the local situation. Second, there are missing data, especially at six and 12 months after completing chemotherapy. Missings were equally distributed among scalp-cooled and non-scalp-cooled patients, and we do not expect patients with missing data to have had a worse or better general QoL, because of the fairly homogenous group of patients. Missing imputation was used to account for the missings. Third, differences in wig purchasing between scalp-cooled and non-scalp-cooled patients might be somewhat biased by the inclusion of 44% of the non-scalp-cooled patients in hospitals that offered scalp cooling. Since these patients did not choose to prevent alopecia by scalp cooling, they might have been less concerned about their appearance. Therefore their QoL might be less influenced by hair loss and they might spend less on head covers and wigs, which might have resulted in an underestimation of the cost-effectiveness of scalp cooling.
This is a first effort to study cost-effectiveness of scalp cooling, which will be subject to change in the near future, while there is certainly much room for improvement. Costs will decrease when scalp-cooled patients delay wig purchase and when patients are more accurately selected for scalp cooling. A high success rate of scalp cooling is needed to attain adequate cost-effectiveness, which will differ per patient group with a certain chemotherapy type and dosage. The rather low costs may be an extra incentive for oncological professionals to offer scalp cooling as a generally highly appreciated service to their patients.
Acknowledgements
Special thanks go to all patients who participated in the study: M. Peerbooms and A. Willemse who assisted in data collection and control, A. Krol, MD, PhD, at the Leiden University Medical Center, who was willing to function as an independent advisor and to answer patients’ questions. Furthermore, we thank the nurses and doctors who performed the study in the following hospitals: A. Schweitzer, Dordrecht; AvL/NKI, Amsterdam; Elkerliek, Helmond; Gelre, Apeldoorn; Havenziekenhuis, Rotterdam; Leids Universitair Medisch Centrum; Máxima Medisch Centrum, Eindhoven; Medisch Centrum Alkmaar; Mesos Medisch Centrum, Utrecht; Refaja, Stadskanaal; St. Lucas, Winschoten; Slingeland, Doetinchem; Spaarne, Hoofddorp; Twee Steden, Tilburg; Ziekenhuis Zevenaar.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by ZonMw (grant number 945-07-002), the Netherlands organisation for health research and development. Author LP was supported by a Cancer Research Award from the Dutch Cancer Society (#UVT-2009-4349).
References
- Hurk van den CJ, Mols F, Vingerhoets AJ, Breed WP. Impact of alopecia and scalp cooling on the well-being of breast cancer patients. Psycho-oncology 2010;19:701–9.
- Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 2002;95:155–63.
- Munstedt K, Manthey N, Sachsse S, Vahrson H. Changes in self-concept and body image during alopecia induced cancer chemotherapy. Support Care Cancer 1997;5:139–43.
- Mulders M, Vingerhoets A, Breed W. The impact of cancer and chemotherapy: Perceptual similarities and differences between cancer patients, nurses and physicians. Eur J Oncol Nurs 2008;12:97–102.
- Macquart-Moulin G, Viens P, Bouscary ML, Genre D, Resbeut M, Gravis G, et al. Discordance between physicians’ estimations and breast cancer patients’ self-assessment of side-effects of chemotherapy: An issue for quality of care. Br J Cancer 1997;76:1640–5.
- Sukel MP, van de Poll-Franse LV, Nieuwenhuijzen GA, Vreugdenhil G, Herings RM, Coebergh JW, et al. Substantial increase in the use of adjuvant systemic treatment for early stage breast cancer reflects changes in guidelines in the period 1990–2006 in the southeastern Netherlands. Eur J Cancer 2008;44:1846–54.
- Breed WPM, Hurk van den CJG, Peerbooms M. Presentation, impact and prevention of chemotherapy-induced hair loss; scalp cooling potentials and limitations. Exp Rev Dermatol 2011;6:109–25.
- Grevelman EG, Breed WP. Prevention of chemotherapy- induced hair loss by scalp cooling. Ann Oncol 2005;16: 352–8.
- Auvinen PK, Mahonen UA, Soininen KM, Paananen PK, Ranta-Koponen PH, Saavalainen IE, et al. The effectiveness of a scalp cooling cap in preventing chemotherapy-induced alopecia. Tumori 2010;96:271–5.
- Wang J, Lu Z, Au JL. Protection against chemotherapy- induced alopecia. Pharm Res 2006;23:2505–14.
- Hussein AM. Chemotherapy-induced alopecia: New developments. South Med J 1993;86:489–96.
- Trueb RM. Chemotherapy-induced alopecia. Sem Cut Med Surg 2009;28:11–4.
- Benjamin B, Ziginskas D, Harman J, Meakin T. Pulsed electrostatic fields (ETG) to reduce hair loss in women undergoing chemotherapy for breast carcinoma: A pilot study. Psycho-oncology 2002;11:244–8.
- Shiao TK, Shiao TK, Shiao JC. Successful treatment of chemotherapy-induced alopecia with LaserCap: A case report. Hair Transplant Forum Int 2010;156.
- Hurk van den CJG, Breed WPM, Mols F. Chemotherapy-induced hair loss. In: Preedy Ve, editors. Handbook of hair in health and disease. Wageningen, The Netherlands: Wageningen Academic Publishers; 2012. p. 403–16.
- Statistics Netherlands. Consumer price index. April 2010. [cited 2010 Sept 09]. Available from: www.cbs.nl.
- Oostenbrink JB, Bouwmans CAM, Koopmanschap MA, Rutten FFH. Handleiding voor kostenonderzoek. Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. 2004 [in Dutch].
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42: 851–9.
- Brazier JE, Rowen D, Mavranezouli I, Tsuchiya A, Young T, Yang Y, et al. Developing and testing methods for deriving preference-based measures of health from condition-specific measures (and other patient-based measures of outcome). Health Tech Assess 2012;16:1–114.
- McDonough CM, Tosteson AN. Measuring preferences for cost-utility analysis: How choice of method may influence decision-making. Pharmacoeconomics 2007;25:93–106.
- Stiggelbout AM, Eijkemans MJ, Kiebert GM, Kievit J, Leer JW, De Haes HJ. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off?Int J Tech Assess Health Care 1996;12:291–8.
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681–94.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: An example using data from a trial of management strategies for atrial fibrillation. BMC Health Services Res 2006;6:52.
- Council for Public Health and Health Care (Raad voor de Volksgezondheid en Zorg). Sensible and sustainable care. 2006.
- Smulders YM, Thijs A. [The cost per year of life gained: Trends and internal contradictions]. Ned Tijdschr Geneeskd 2006;150:2467–70.
- Bernard M, Brignone M, Adehossi A, Pefoura S, Briquet C, Chouaid C, et al. Perception of alopecia by patients requiring chemotherapy for non-small-cell lung cancer: A willingness to pay study. Lung Cancer 2011;72:114–8.
- Hurk van den CJG, Akker van den-van Marle ME, Breed WPM, Poll van de-Franse LV, Nortier JWR, Coebergh JWW. Impact of scalp cooling on chemotherapy-induced alopecia, wig use and hair growth of patients with cancer. J Oncol Nurse 2013 (in press).
- Hurk van den CJ, Peerbooms M, van de Poll-Franse LV, Nortier JW, Coebergh JW, Breed WP. Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients – results of the Dutch Scalp Cooling Registry. Acta Oncol 2012;51:497–504.
- Hurk van den CJ, Breed WP, Nortier JW. Short post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer 2012;20:3255–60.
- Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ 2004;13:873–84.
- Lamers LM, McDonnell J, Stalmeier PF, Krabbe PF, Busschbach JJ. The Dutch tariff: Results and arguments for an effective design for national EQ-5D valuation studies. Health Econ 2006;15:1121–32.