Abstract
Background. As prostate cancer (PC) mortality reduction results are not unequivocal, a special emphasis has to be put on other aspects of the prostate-specific antigen (PSA) screening, including effects on quality of life. In the present study we describe the short-term effects of various phases of PC screening on health-related quality of life (HRQL). Material and methods. The study participants were randomized into the screening arm within the Finnish component of the European Randomized Study on Screening for Prostate Cancer (ERSPC). The RAND 36-Item Health Survey on HRQL and questionnaires on sociodemographic and behavioral factors were delivered to participants at various phases of the first screening round: 1) 500 participants at invitation; 2) 500 after screening; 3) 500 after obtaining the PSA result; 4) to 300 participants after undergoing digital rectal examination (DRE) (but prior to being informed of its result); and 5) approximately 300 after prostate biopsy. At each stage, a new sample of participants was recruited. Results. Response rates were 59% at invitation, 77% after PSA blood test, 54% after PSA result and 69% after DRE. The men recruited at each stage were comparable in respect to socioeconomic variables. The HRQL scores in RAND-36 subscales showed little variation in the different phases of the screening process. Compared with the previous phase, the social function score was slightly lower after obtaining the PSA result than after blood test, the emotional role score lower after DRE than after PSA result and the pain-related score lower after DRE than after TRUS and biopsy. The screening participants were comparable to the general population as their HRQL scores were similar to an age-stratified general Finnish male population. Conclusion. Short-term HRQL effects of prostate cancer screening appear minor and transient.
Prostate cancer (PC) is the most common cancer and third most common cause of cancer death in men in the Western world [Citation1]. As PC is a major public health issue and prostate-specific antigen (PSA) has been demonstrated as a good biomarker of the disease, major research efforts have focused on the effectiveness of a population-based PSA screening. The recently published randomized trials have reported relatively controversial results of screening on PC mortality [Citation2–7].
As the results on mortality reduction are not unequivocal and the absolute mortality reduction appears small or moderate in size, a special emphasis has to be put on other aspects of the screening process, including its effect on the physical and mental well-being. Screening can adversely affect the participants’ health-related quality of life (HRQL), but the literature is scarce [Citation8]. A previous prospective study concluded that screening participants did not experience substantial short-term impact on health status despite short-lasting side effects related to prostate biopsy [Citation9]. Nor have men undergoing PSA testing shown any significant increase in anxiety, even with an abnormal PSA results [Citation9,Citation10]. Men cope well with the testing process even in the case of an abnormal PSA test result, although a minority of them experience elevated distress at the time of biopsy which is not entirely resolved by a negative result [Citation11]. Men with a disposition to anxiety have also been shown to experience higher levels of anxiety than others throughout the screening process [Citation9].
In the present study we tried to evaluate how men managed in different screening interventions. The aim was to quantify the short-term impact of the PSA screening process on HRQL.
Material and methods
Procedure
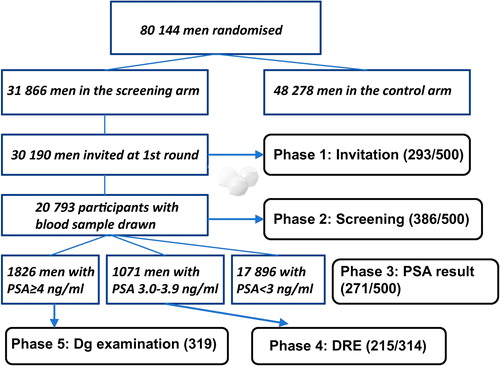
The detailed protocols for the ERSPC trial and its Finnish arm have been described elsewhere [Citation12,Citation13]. In Finland, for the first screening round between the years 1996 and 1999 approximately 80 000 men were identified from the Finnish population registry and randomized into screening (31 866) or control arm (48 278). The target population consisted of men born from 1929 to 1944 and living in the metropolitan areas of Helsinki and Tampere. Men with a previous diagnosis of PC (identified from the Finnish Cancer Registry) as well as those who had emigrated or deceased prior to invitation were excluded. A blood sample was drawn from the participants (20 793 men, 69% of the 30 190 men invited). Men with a PSA concentration between 3.0 and 3.9 ng/ml were initially referred to a digital rectal examination (DRE) by an urologist, and since 1998 determination of the free/total PSA ratio was used as an ancillary test in these men. Men with PSA > 4 ng/ml were referred to a full clinical examination, while DRE was used as an ancillary standalone test for men with marginally elevated PSA. Those with a PSA ≥ 4 ng/ml or a suspicious finding in DRE or free/total PSA ratio < 0.17 were referred to diagnostic examination comprising a transrectal ultrasound (TRUS) with prostate biopsy.
Materials
For the purposes of the present study, men attending screening were asked to fill in questionnaires on sociodemographic and lifestyle factors and general HRQL (RAND 36-item Health Survey). The study cohort represents a sample from the first round of the Finnish arm of the ERSPC trial with men invited to participate: 1) 500 participants at invitation; 2) 500 after drawing a PSA blood sample; 3) 500 after receiving the PSA result; 4) 314 after undergoing DRE (prior to being informed of its result, but aware of the PSA result); and 5) > 300 after diagnostic examination, i.e. TRUS and biopsy (participants unaware of the result, but being informed of the PSA result) (). The exact number of questionnaires delivered after TRUS and biopsy is not known because of additional copies of the questionnaires being made and delivered without keeping track of the numbers at one participating department. The study participants were different in all five screening phases.
HRQL questionnaires were delivered to a total of more than 2000 screening participants at five phases of the first screening round. In total 293 (59%, four subjects excluded due to incompletely filled questionnaires) responded at invitation, 386 (77%, nine exclusions) at screening, 271 (54%, 12 exclusions) at PSA result and 217 (69%, six exclusions) at DRE. The exact number of questionnaires delivered after TRUS and biopsy is not known, but the number of evaluated responders for this phase was 319. The item non-response in the questionnaires was 0.5–1.5% at different screening steps and 8.8–12.5% of screening attendants failed to respond to one or more questions.
Men received an information letter about the procedures of the study and general information about PC. The initial questionnaires were delivered with the invitation letter. The HRQL assessments at the two last phases were performed before the responders knew the DRE or biopsy results.
RAND 36 – Item Health Survey
The RAND-36 questionnaire consists of a total of 36 items divided into eight subscales: physical functioning, bodily pain, role limitations due to physical/emotional problems, emotional well-being, social functioning, energy/fatigue and general health perceptions. All eight subscales are separately scored from 0 to 100 and a higher score indicates a better HRQL. The RAND 36-Item Health Survey [Citation14] contains exactly the same questions as the MOS SF-36 [Citation15], but the scoring of the general health and bodily pain differs slightly.
The Finnish version of the RAND 36-Item Health Survey has been validated and has good reproducibility in the Finnish population (Cronbach's α 0.80–0.94). To allow comparison to the general population, age-stratified reference values for the subscales have been established [Citation16]. In this study group, Cronbach's alpha for each item was close to 0.8 (range 0.73–−0.89).
Operationalization of psychosocial variables
Data on sociodemograhic and behavioral factors (age, marital status, education, employment, position, smoking, exercise) were collected at the initial stages of screening but not from screen-positive men. In the survey the level of education was divided to a primary (9 years) to secondary (10–12 years) school, vocational training and higher education (college or university). The employment status (full/part-time employed, unemployed, retired) and socioeconomic status (higher, lower, retired) were both divided into three categories. Also information on marital (unmarried, married/cohabitation, divorced, widow) and smoking status (yes/no) was gathered. Physical exercise was divided into five categories describing physical activity of a participant (exercise not at all, occasionally, once/twice/at least three times a week).
Statistics
For statistical analyses SPSS v.17.0 (Chicago, IL, USA) was used. In all tests, the level of statistical significance was set at p = 0.05. A non-paired t-test or non-parametric Mann-Whitney U-test was used to compare the RAND-36 scores between the five samples of screening participants, depending on whether the assumption about a normal distribution was met or not. The RAND-36 mean scores in eight subgroups were also compared to an age-stratified general Finnish male population (60–64 years). Associations between eight HRQL variables and assumed HRQL predicting factors (age, socioeconomic status, PSA-value) were analyzed using ordinal logistic regression. For the ordinal logistic regression analysis, the RAND-36 scores in subgroups were divided into quartiles (cutpoints set at 25th, 50th and 75th percentiles) and the response variable was the grouped HRQL result (the reference being the lowest quartile with the odds ratio representing increment by a quartile, i.e. increase to the next group with the higher HRQL score). The associations between eight HRQL variables and potential explanatory factors (e.g. age, PSA, sociodemographic and behavioral factors) were also analyzed using linear regression (with the subscale score as the response variable) and the results were consistent with those obtained by the logistic analysis.
Ethics
The study protocol was approved by the ethics committee of each participating hospital and written informed consent was obtained from all participants.
Results
The mean age of the participants in the different phases of the screening process was 60–63 years. Information on sociodemographic and behavioral factors was collected at phases 1–3 (invitation, attendance and partly at PSA result group), but not at ancillary screening test (DRE) or diagnostic examination (TRUS/biopsy). No major differences in the sociodemographic or behavioral factors were observed between the participants at various stages of the screening process ().
Table I. Sociodemographic and lifestyle factors among subjects included at various phases of the screening program. Information on marital status, level of education and physical exercise were not collected at the time when men were informed of the screening results.
Of the various HRQL scores, those related to physical and emotional role, and physical and social function tended to show the highest scores (medians 90–100) (). Consistently at each phase, the lowest scores were found for general health (median 65), followed by energy/fatigue. No clear and systematic changes could be found during the screening process. The only decrease by at least six points was in the role-emotional subscale at DRE. A small but statistically significant decrease was also observed in social function after receiving the PSA result. The pain-related HRQL score was higher after TRUS and biopsy than at previous phase. When participants of the PSA result group with an abnormal screening result (PSA ≥ 3) were compared to those with normal PSA, unexpectedly, HRQL scores were even higher in men with an abnormal PSA value. Comparing these two groups, no significant differences were observed between mental and general health dimensions.
Table II. Health-related quality of life as RAND-36 scores (all eight subscales) (median, 25th–75th percentiles) at five different screening phases and the reference values of the general Finnish male population (aged 60–64). Comparison is always performed with the previous phase of the screening programme. Statistically significant changes in bold.
The results of the ordinal logistic regression were largely unremarkable, as significant associations (p < 0.01) were detected for age with poorer physical function, but improved energy and mental health; physical exercise with better physical function, energy and general health; as well as education with physical function and role-physical. Among the findings of borderline significance (particularly considering multiple comparisons) was an unexpected association of serum PSA level with better general health (but lower pain score) at DRE, but not other phases.
Compared to the reference values obtained from the general Finnish male population aged 60–64, HRQL among screening participants was similar or slightly higher, though no significant differences emerged.
Discussion
The population-based PC screening process did not substantially affect the short-term HRQL as assessed by the RAND-36 instrument. Only minor differences were seen in participants’ HRQL at various phases of the screening. HRQL effects appeared transient as changes occurred only at one point in time and not persisted throughout the screening episode. The mean HRQL scores were also comparable to the general Finnish population.
Concern has been expressed about the effects of the screening process on participants’ quality of life (QoL) and psychological health. Screening does not appear to have negative emotional impacts in longer term [Citation17] and some previous studies have concluded that the screening process has little if any impact on participant's psychological health [Citation10,Citation18]. In cancer-related anxiety, increased short-term distress, but no severe or long-term effects have been reported [Citation19]. However, participants with pre-existing anxiety tend to remain anxious [Citation9]. In the Rotterdam section of the ERSPC trial, PC screening did not induce important short-term effects on health-status or QoL, despite short-lasting side effects related to the prostate biopsy [Citation9]. Our study results support this finding, with a larger material for the final stages of the screening episode. Only a minority of PC screening participants experience elevated distress at the time of biopsy and after a negative result [Citation11]. Nor does older age, a positive family history or a higher PSA level predict anxiety in men during testing for PC [Citation20]. Even men with initially false-positive screening tests tend to perceive their screening experience as positive [Citation21]. In contrast, men with a benign biopsy are concerned about PC [Citation22,Citation23]. Overall it appears that substantial unfavorable psychological and health-related effects occur mainly after cancer diagnosis.
In the present study, the participants were aware of their abnormal screening results, i.e. an abnormal PSA value (the PSA result, DRE, TRUS/biopsy groups) or suspicious DRE finding (TRUS/biopsy), but were not informed of the findings at the current examination when the HRQL was evaluated. Hence, these points were assumed to represent the most stressful and anxiety generating stages of the screening process. Unexpectedly HRQL scores were even higher in men with an abnormal screening result (PSA ≥ 3) compared to those with a normal value after obtaining the PSA result. It is possible that men with an abnormal screening result are relieved that a higher PSA value was noticed indicating further clinical examinations.
No substantial differences were observed between the five stages of screening with few exceptions. The score for the role-emotional subscale was reduced following DRE as ancillary screening test, but it is unclear if this can be interpreted as a direct effect of the test. It is conceivable, however, that it might be due to an increased anxiety among men referred to further diagnostic examinations. Higher HRQL scores after diagnostic examination is likely attributable to chance (relief due to a negative biopsy result would be likely reflected on other subscales). This possibility was further supported when considering multiple comparisons – eight scales were compared between two assessments a total of four times each (with a likelihood of observing at least one significant difference once of 0.81, obtained as 1–0.9532).
In the ordinal regression analysis, a higher PSA predicted better HRQL, except at DRE where it was associated with lower scores in pain subscale. Previous studies have shown that men are not able to assess the probability of false-negative or -positive results following an elevated PSA [Citation24] and men with abnormal screening test results cannot judge accurately the risk of PC, both overestimation and underestimation are common [Citation23,Citation25]. And not only for patients, abnormal screening test results may also be a challenge to a physician to judge the actual risk of prostate cancer on the basis of a single PSA value.
Our study was based on independent samples of men recruited at various stages of the screening program. This design was chosen to avoid bias from loss to follow-up, which was deemed likely if the same group of men were asked to fill out the study questionnaire up to four times at consecutive phases. Also, a large number of men would have been needed in order to make sure that a sufficient sample size would have been available even in the final phase (due to incomplete participation and relatively low proportion of men referred and undergoing biopsy). Finally, another potential bias could have resulted from men responding in an identical fashion ‘out of habit’. However, the current approach does increase sampling error, i.e. the samples could differ in their baseline characteristics. We found, however, no indication of such underlying differences, when we compared three of the samples in terms of sociodemographic and lifestyle factors.
In the present study we used a generic HRQL instrument that has been extensively validated and used earlier. One challenge in measuring HRQL is its’ character as a multidimensional construct incorporating person's mental, physical and social components. To assess HRQL effects, generic, domain- or disease-specific questionnaires can be used. Generic questionnaires such as RAND-36 allow comparisons between men with and free of a condition, as well as across diseases and between disease stages. One problem with generic HRQL questionnaires may be that they are not sensitive and specific enough to detect relatively subtle changes in man's physical and emotional well-being induced by the PSA screening. Measuring generic HRQL is only an indirect way of studying those possible arisen emotional reactions. RAND 36/SF-36 may not ideally measure specific responses such as fear or anxiety. Also, these questionnaires contain items not directly relevant for the screening process. Our findings nevertheless provide evidence supporting the notion that the screening process does not cause major QoL changes.
The mean HRQL scores among screening participants were observed to be higher than in general Finnish population. This is due to selection, as screening participants are likely to be more educated and in better health than non-participants (phenomenon known as healthy screenee bias). There may also be further selection among the screened men so that the healthier men may have been participated in the survey. Further, the age distributions do not match perfectly but the reference values are from men aged 60–64 years, while our age range is wider particularly toward younger ages (55–67) [Citation16]. Also, there may be geographical differences, as the screening trial is based on the two largest metropolitan areas in Finland. The most significant differences appeared to be in social function and physical/emotional role; the first probably representing activity and the latter ability to participate in screening. These HRQL subscales can be suitable to separate participants versus non-participants and so predict participating in screening.
In conclusion, PC screening as carried out in the Finnish arm of the ERSPC study has no substantial impact on short-term QoL. In the future, more effort is needed for evaluation of long-term effects of screening, as well as QoL among men with screen-detected cancers, relative to other men with PC within the screening trial.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by grants from the Finnish Cancer Organisations and Academy of Finland (grant no. 123054 and 260931).
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127: 2893–917.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366:981–90.
- Roobol MJ, Kerkhof M, Schröder FH, Cuzick J, Sasieni P, Hakama M, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur Urol 2009;56:584–91.
- Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11:725–32.
- Bul M, Schröder FH. Screening for prostate cancer – the controversy continues, but can it be resolved?Acta Oncol 2011;50(Suppl 1):4–11.
- Berg CD. The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial: The prostate cancer screening results in context. Acta Oncol 2011;50(Suppl 1):12–7.
- Andriole GL, Crawford ED, Grubb RL 3rd,Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104:125–32.
- Ilic D, O’Connor D, Green S, Wilt TJ. Screening for prostate cancer: An updated Cochrane systematic review. Br J Urol Int 2011;107:882–91.
- Essink-Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schröder FH. Short-term effects of population-based screening for prostate cancer on health-related quality of life. J Natl Cancer Inst 1998;90:925–31.
- Brindle LA, Oliver SE, Dedman D, Donovan JL, Neal DE, Hamdy FC, et al. Measuring the psychosocial impact of population-based prostate-specific antigen testing for prostate cancer in the UK. Br J Urol Int 2006;98: 777–82.
- Macefield RC, Metcalfe C, Lane JA, Donovan JL, Avery KN, Blazeby JM, et al. Impact of prostate cancer testing: An evaluation of the emotional consequences of a negative biopsy result. Br J Cancer 2010;102:1335–40.
- Schröder FH, Denis LJ, Roobol M, Nelen V, Auvinen A, Tammela T, et al. The story of the European Randomized Study of Screening for Prostate Cancer. Br J Urol Int 2003;92(Suppl 2):1–13.
- Finne P, Stenman U-H, Määttänen L, Mäkinen T, Tammela TL, Martikainen P, et al. The Finnish trial of prostate cancer screening: Where are we now?Br J Urol Int 2003;92(Suppl 2):22–6.
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ 1993;2:217–27.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83.
- Aalto AM, Aro AR, Teperi J. RAND-36 as a measure of health-related quality of life: Reliabilty, construct validity and reference values in the Finnish general population. Helsinki: Stakes; 1999.
- Collins RE, Lopez LM, Marteau TM. Emotional impact of screening: A systematic review and meta-analysis. BMC Public Health 2011;11:752.
- Awsare NS, Green JSA, Aldwinckle B, Hanbury DC, Boustead GB, McNicholas TA. The measurement of psychological distress in men being investigated for the presence of prostate cancer. Prostate Cancer Prostatic Dis 2008;11:384–9.
- Wardle FJ, Collins F, Pernet AL, Whitehead MI, Bourne TH, Campbell S. Psychological impact of screening for familial ovarian cancer. J Natl Cancer Inst 1993;85:653–7.
- Macefield RC, Lane JA, Metcalfe C, Down L, Neal DE, Hamdy FC, et al. Do the risk factors of age, family history of prostate cancer or a higher prostate specific antigen level raise anxiety at prostate biopsy?Eur J Cancer 2009;45: 2569–73.
- Essink-Bot ML, Korfage IJ, De Koning HJ. Including the quality-of-life effects in the evaluation of prostate cancer screening: Expert opinions revisited?Br J Urol Int 2003; 92(Suppl 2):101–5.
- Fowler FJ, Barry MJ, Walker-Corkery B, Caubet J-F, Bates DW, Lee JM, et al. The impact of a suspicious prostate biopsy on patients’ psychological, socio-behavioural, and medical care outcomes. J Gen Intern Med 2006;21:715–21.
- Katz DA, Jarred DF, McHorney CA, Hillis SL, Wiebe DA, Fryback DG. Health perceptions in patients who undergo screening and workup for prostate cancer. Urology 2007;69: 215–20.
- Chan EC, Vernon SW, O’Donnell FT, Ahn C, Greisinger A, Aga DW. Informed consent for cancer screening with prostate-specific antigen: How well are men getting the message?Am J Public Health 2003;93:779–85.
- Frosch DL, Kaplan RM, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med 2001;16:391–8.