Abstract
Background. Survival from breast cancer (BC) in Estonia has been consistently among the lowest in Europe. The aim of this study was to examine most recent trends in BC survival in Estonia by age and stage. The trends in overall BC incidence and mortality are also shown in the paper. Material and methods. Estonian Cancer Registry data on all cases of BC, diagnosed in women in Estonia during 1995–2007 (n = 7424) and followed up for vital status through 2009, were used to estimate relative survival ratios (RSR). Period hybrid approach was used to obtain the most recent estimates (2005–2009). Stage was classified as localized, local/regional spread or distant. Results. BC incidence continued to rise throughout the study period, but mortality has been in steady decline since 2000. The distribution of patients shifted towards older age and earlier stage at diagnosis. Overall age-standardized five-year RSR increased from 63% in 1995–1999 to 74% in 2005–2009. Younger age groups experienced a more rapid improvement compared to women over 60. Significant survival increase was observed for both localized and locally/regionally spread BC with five-year RSRs reaching 96% and 70% in 2005–2009, respectively; the latest five-year RSR for distant BC was 11%. Survival for T4 tumors was poor and large age difference was seen for locally/regionally spread BC. Conclusions. Considerable improvement in BC survival was observed over the study period. Women under 60 benefited most from both earlier diagnosis and treatment advances of locally/regionally spread cancers. However, the survival gap with more developed countries persists. Further increase in survival, but also decline in BC mortality in Estonia could be achieved by facilitating early diagnosis in all age groups, but particularly among women over 60. Investigations should continue to clarify the underlying mechanisms of the stage-specific survival deficit in Estonia.
At the end of the 20th century, relative survival from breast cancer (BC) improved steadily in all European countries, although at different rates, with the gap between eastern and western European countries widening [Citation1]. These geographical differences appeared to persist also in the first decade of the 21st century [Citation2]. Survival from BC in Estonia has been consistently among the lowest in Europe. According to the EUNICE survival analysis, a gap of over 20% in the five-year relative survival was evident in 2000–2004 between Estonia and Geneva, the registry with the highest survival [Citation2]. Projections for 2005–2009 suggested that the survival would reach almost 90% in many registries in Europe while it would remain around 70% in Estonia [Citation2]. According to the results from the EUROCARE high resolution study (1990–1992), the relative excess risk of death among BC patients in Estonia could not be completely explained by later stage at presentation [Citation3].
The aim of this population-based study was to examine the most recent BC survival trends in Estonia by age and stage, and to attempt to identify the main contributors to the persisting survival disparity in Estonia. To our knowledge, stage-specific survival in Estonia has not been reported previously. In addition to survival analysis, the trends in overall BC incidence and mortality are presented in the paper.
Material and methods
The study used data collected by the Estonian Cancer Registry (ECR), a population-based registry that covers the whole country (population 1.34 million in 2009) and has had complete nationwide coverage since 1968 [Citation4]. Mortality trend was calculated based on data from the WHO Mortality Database [Citation5]. For survival analysis, data on all cases of invasive BC (ICD-O-2 topography codes C500–C509) diagnosed in women in Estonia during 1995–2007 (n = 7453) were obtained from the ECR, regardless of cancer sequence. After the exclusion of death certificate only and autopsy cases (n = 29), 7424 cases were included (four of them were second primary BCs). The notification forms used for data collection were uniform during the whole study period. The patients were followed through 2009 by linkage to the Estonian Population Registry. The vital status of the patients as well as the date of death/emigration was ascertained. The extent of disease is coded in the ECR database as localized, regional, direct extension into surrounding tissues, distant, advanced NOS, and unknown. Pathological or clinical TNM stage is not available from the registry database in coded format, but it is reported by clinicians on the notification form according to current TNM classification and entered in text format. For this study, all cases were reviewed and summary stage was compiled based on TNM classification: localized (T1–3 N0 M0); local/regional spread (T1–3 N1–3 M0 or T4 Nany M0) or distant (Tany Nany M1). In some cases where T or N was not available, summary stage was derived from the reported TNM-based stage grouping (e.g. stage I – localized). Summary stage was checked against the extent of disease and the original notification form was consulted if necessary. Category “unknown” includes cases with no or incomplete information on stage.
Relative survival ratios (RSR) were calculated as the ratio of the observed survival of the cancer patients and the expected survival of the underlying general population. The latter estimate was calculated according to the Ederer II method [Citation6] using national life tables for female population stratified by single year of age and calendar year. We used traditional cohort analysis for patients diagnosed in 1995–1999, 2000–2004 and 2005–2007, and period estimation for 2005–2009 in order to detect the most recent survival trends [Citation7]. Period estimates are considered to be very similar to actual survival rates observed in the future for patients who were at risk and diagnosed during this specific period [Citation8]. As follow-up was more up-to-date than registration of incident cases, a modification of period analysis, called hybrid analysis was used [Citation9].
The International Cancer Survival Standard (ICSS) population was used for age-standardizing overall survival estimates [Citation10]. All calculations were conducted with Stata 12.1 (StataCorp LP, Texas USA), survival analysis was performed using the strs module [Citation11].
The study was approved by the Tallinn Medical Research Ethics Committee.
Results
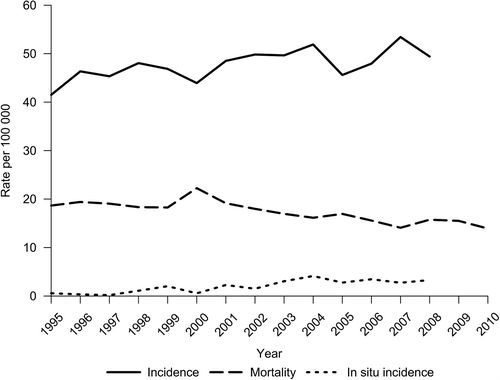
presents the age-standardized incidence (in situ and invasive) and mortality rates of female BC in Estonia from 1995 until the most recent year available. While the incidence of invasive BC continued to rise throughout the study period, a steady decline in mortality has been apparent since 2000. The incidence of in situ BC increased from 0.6/100 000 in 1995 to 3.3/100 000 in 2008.
Figure 1. Female breast cancer incidence and mortality in Estonia (age-standardized to world standard population), 1995–2010.
The survival analysis included 7424 cases of invasive BC diagnosed in 1995–2007. Overall, 96% of the cases were microscopically confirmed (). Median age at diagnosis was 60 years (range 22–96 years).
Table I. Distribution of cases by age at diagnosis, stage at diagnosis and period of diagnosis: women diagnosed with breast cancer in Estonia, 1995–2007.
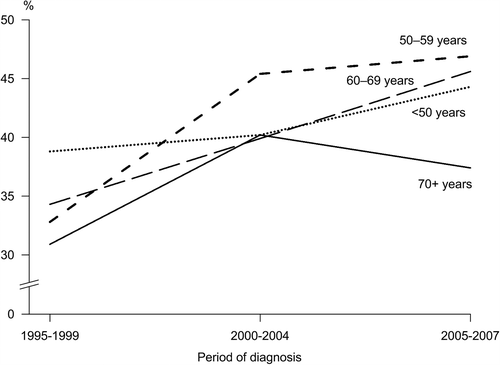
About half of the patients (52%) were 60 years or older at the time of diagnosis and the distribution of patients tended to change towards older age groups during the study period. In stage distribution, there was a clear shift towards earlier stage (). The proportion of localized disease increased from 34% in 1995–1999 to 43% in 2005–2007 (p < 0.005) (). The rise was steepest among women age 50–59 at diagnosis (from 33% to 46%; p < 0.005). Substantial variation in stage distribution was seen across age groups: during the most recent period of diagnosis, 2005–2007, the proportion of distant cases was 4%, 5%, 9% and 11% among women age < 50, 50–59, 60–69 and 70 + years, respectively; similar variation was apparent for locally advanced (T4) tumors: 5%, 6%, 7% and 10%, respectively (the difference between the oldest and youngest age group was statistically significant for both distant and T4 cases; p < 0.005). There was a significant increase in the proportion of small (T1) tumors over the study period (p < 0.0005) while the proportion of T4 tumors decreased (p < 0.0005).
Figure 2. Percentage of cases diagnosed at localized stage among all breast cancer cases in Estonia by age at diagnosis, 1995–2007.
Cumulative relative survival curves up to 10 years are presented in . Overall five-year RSR in 2005–2009 was 76% (age-adjusted 74%), demonstrating a significant increase of almost 12% over the study period (). As younger age groups experienced a more rapid improvement compared to women over 60 years of age, the age difference in survival increased. Significant survival increase was observed for both localized and locally/regionally spread BC with five-year RSRs reaching 96% and 70%, respectively (, ), although the RSR for locally advanced T4 tumors remained very low. A significant increase was also observed for 10-year survival from 1995–1999 (RSR 54.0, 95% CI 51.7–56.4; age-adjusted RSR 49.8, 95% CI 46.1–53.4) to 2005–2009 (RSR 66.2, 95% CI 63.9–68.6; age-adjusted RSR 63.1; 95% CI 59.0–67.0). The trends observed for 10-year RSRs by age and stage were similar to those seen for five-year RSRs.
Figure 3. Age-standardized cumulative relative survival from breast cancer in Estonia, 1995–2009. Cohort estimates for 1995–1999, 2000–2004 and 2005–2007, period estimates for 2005–2009.
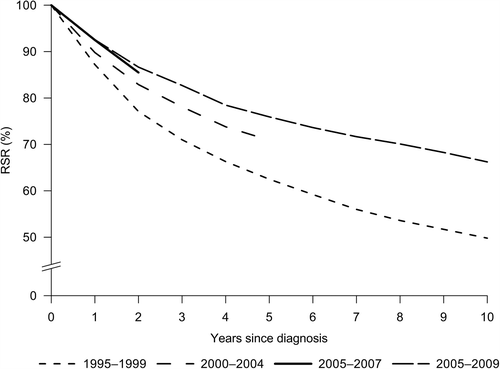
Figure 4. Stage-specific cumulative relative survival from breast cancer in Estonia, 1995–2009. Cohort estimates for 1995–1999 and 2000–2004, period estimates for 2005–2009.
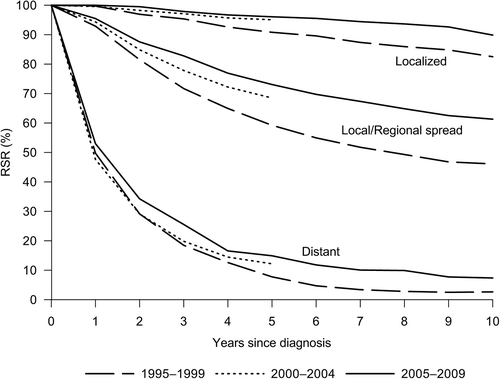
Table II. Relative survival from breast cancer in Estonia, 1995–2009.
presents age-specific period estimates of five-year survival for 2005–2009 by stage and tumor size. While the estimates across age groups were quite homogenous for localized stage, remarkable differences were seen for non-localized disease. A 16% difference was evident between the youngest and oldest age groups for locally/regionally spread cancer. Patients with T4 tumors experienced very poor survival, particularly women age 70 and over.
Table III. Five-year relative survival from breast cancer by stage, tumor size and age in Estonia, 2005–2009.
Discussion
In this population-based study, we observed marked improvement in BC survival in Estonia from 1995−1999 to 2005−2009, with an absolute rise both in five- and 10-year RSRs of about 12%. In a previous analysis, 10% survival increase was observed over a 20-year period from mid-1970s to mid-1990s [Citation12]. The rising BC incidence and survival rates along with decreasing mortality observed in this study are consistent with trends shown for many countries of Europe [Citation13]. Although BC incidence in Estonia remains lower than in western-European countries, an upward trend has been evident for more than 20 years and it is likely associated with the same determinants as suggested for other European countries [Citation13]. In Estonia, the increasing impact of reproductive risk factors, but also other factors that may be more amenable to intervention, such as obesity and low physical activity, is probably related to the gradual adoption of western lifestyle.
In our analysis, BC survival increased in each age group and stage category, but the extent of the increase varied. Stage was a highly important prognostic factor as also reported elsewhere [Citation14,Citation15]. In the 1990–1992 EUROCARE high resolution study, the five-year survival indices for localized disease (T1–3 N0 M0) in Estonia were 90% or higher, similarly to most other participating European registries [Citation3]. Improvement continued throughout our study period and the 2005–2009 estimate reached 96%. Within localized stage category, variation of survival by age was very small, suggesting overall excellent care. The proportion of patients diagnosed with localized disease increased 1.3-fold during our study period, and alongside with that, the proportion of early tumors (T1) also steadily increased. However, diagnosing BC at localized stage is still less frequent compared to other countries. For example, localized tumors accounted for 50% and 63% of all cases with available stage information in 2005–2008 in Saarland and USA (SEER regions), respectively [Citation15], versus 44% in Estonia in 2005–2007 (). The proportion of early tumors was the lowest among patients age 70 years and over, but their high five-year RSR indicates that outcome for localized BC was overall good irrespective of age. Thus, an increased proportion of early cases with excellent survival can be considered as the main contributor to the rise in overall BC survival in Estonia. As demonstrated in , women aged 50–59 experienced the largest shift towards earlier diagnosis, which is consistent with screening activities. While organized mass screening for women age 50–59 has been conducted since 2004, it was preceded by two years of BC early detection program in the same age group, where invitations were combined with self-referral, and pilot projects in two larger cities since late 1990s [Citation16]. Only from 2007 onwards, women up to age 62 are included. Unfortunately, the effect of screening is very difficult to estimate as the preparations for establishing a screening registry have started only very recently and accurate screening statistics are not yet available. As an indirect indicator of screening activity, the incidence of in situ tumors has risen steadily during the study period, however, it still remains lower than in the countries with a long history of well-established screening (8.5/100 000 in Finland, e.g. [Citation14]), whether due to under-reporting or under-diagnosis remains to be examined. It should be mentioned that the screening program is available only to women with current health insurance (in 2008, the estimated insurance coverage among adult women in Estonia was 95%) and participation among those invited remains poor (around 50% in 2005–2006), resulting in low examination coverage (39% in 2005–2006) [Citation17]. Besides screening and increased availability of mammography equipment, one cannot ignore the role of increased BC awareness in the shift towards earlier diagnosis since the end of 1990s.
Table IV. Age-adjusted period estimates of five-year relative survival from breast cancer.
As another important finding, a 10% absolute rise of five-year RSR between 1995–1999 and 2005–2009 was seen for locally/regionally spread BC, suggesting advances in treatment. The major contributor to this was survival improvement for T1–3 node-positive cases. In Estonia, consensus guidelines have been adopted and the availability of modern treatments has improved since 1990s. New anticancer drugs have become increasingly available (anthracyclines, taxanes, trastuzumab, aromatase inhibitors, etc.). Despite recent rapid improvement, however, the survival deficit of patients in Estonia with locally/regionally spread BC is still 15% compared to US and 11% compared to Germany (). In this study, particularly low survival was seen for T4 tumors in Estonia. In a CONCORD high-resolution analysis of patients diagnosed with BC during late 1990s, the survival of T4 tumors was markedly lower in Eastern European countries (including Estonia) compared to other regions of Europe and the US [Citation18]. The same study demonstrated that the proportion of women treated with surgery in Estonia was the lowest among participating registries [Citation18]. It was suggested that low healthcare expenditure in Eastern European countries may have had an important effect on the quality of BC treatment, as chemotherapy and hormonal treatment were more widely used than more expensive surgery and radiotherapy [Citation18]. Unfortunately, no data are available regarding recent patterns of care in Estonia, including whether high levels of comorbidity have restricted the choice of therapeutic options or necessitated dose reductions. It has been long known that the effectiveness of adjuvant chemotherapy correlates with optimal doses applied and patients receiving less than 65% of the planned dose do not benefit from chemotherapy [Citation19]. Delay in starting treatment has been shown to affect survival among patients with late-stage disease [Citation20]; waiting times for appointment with cancer specialist or timeliness of surgery or adjuvant therapy have not been studied in Estonia. In addition to patients with locally/regionally spread BC, those with metastatic BC in Estonia also experienced considerably poorer survival compared to other countries (). In a recent Dutch study, patients with distant metastasis at initial presentation were shown to benefit from all therapeutic modalities, including surgery, radiotherapy and first-line systemic therapy, particularly from new targeted therapies [Citation21].
Stage migration should be considered as one possible explanation for stage-specific survival differences. In 1997, only 23% of patients who underwent lymphadenectomy in Estonia had 10 or more lymph nodes examined, which was considerably lower than in most other European countries [Citation18]. It is also possible that insufficient diagnostic work-up fails to find asymptomatic distant metastases at diagnosis and results in poorer survival for both locally/regionally advanced as well as distant cancers. Previous studies have shown lower diagnostic activity among older patients compared with younger women [Citation22]. Besides less accurate diagnosis, it has also been documented that elderly patients are less likely to receive standard management or to have surgery even after adjustment for co-morbidity [Citation23]. Elderly patients in Estonia may have benefited less from treatment advances, as the disparity of survival by age appeared to increase over time. Also, among women age 70 years and over, the proportion of large extensive tumors (T4) appeared to be notably higher compared to younger patients, suggesting delays in seeking cancer care. As demonstrated by a recent study, women of this age group were significantly less likely than younger women to seek appointment immediately after discovering symptoms [Citation24].
Despite steady improvement in BC survival, the period estimate of five-year relative survival for 2005–2009 in Estonia (76%) remained around 10% lower than the estimates recently reported by several other countries/regions (). There is still about a 20-year lag in the five-year RSR compared to Finland [Citation25] and the Estonian estimates have not yet reached the European mean five-year RSR of 79% observed in EUROCARE-4 study for 1995–1999 [Citation26]. Although prognosis for localized BC is excellent in Estonia, BC is still less often diagnosed at localized stage compared to many other countries, particularly among older women. Extension of the upper age limit of the screening program, as recommended [Citation27], would help to improve early diagnosis among women up to age 70. Screening should be made available to all women of target birth cohorts, including uninsured women, in order to prevent socioeconomic differences in BC survival. Our results suggest that part of the observed survival deficit in Estonia can be attributed to poorer outcome for locally/regionally advanced and distant disease. For these patients, the utilization of all indicated options of surgery and adjuvant therapy is an important determinant of patient survival. Supportive care for elderly and frail patients has not been well established in Estonia. Deficits in BC management could be related to several factors, including timeliness of surgery or adjuvant therapies, use of sentinel node biopsy, use of radiotherapy after breast conserving surgery, use of palliative or supportive care options or dose reductions in chemotherapy due to comorbidities, etc. To estimate the adequacy of BC management in Estonia, and to compare patterns of care or the proportion of different prognostic variants of BC such as triple-negative cancers with other countries, a high-resolution approach is warranted, as also highlighted by the CONCORD survival working group [Citation18]. The establishment of a national quality registry would allow continuous monitoring of quality of care.
The results of this study should be considered in light of potential limitations, the most important of which is the limited availability of clinical information. The ECR has no access to data on detection mode (screening vs. symptomatic) and co-morbidity, or to detailed data on diagnostic procedures and cancer treatment. Information on stage was unavailable for 5% and data on tumor size for 10% of the patients. The profile of cases with unknown stage may differ between registries and therefore obscure stage-specific comparisons between countries. The improvement of survival may contain some elements of lead time effect due to increased mammography coverage and the start of mass screening program within the time-frame of the study. The main strength of the study is the use of a population-based cancer registry with sufficient completeness of BC cases as these patients are almost exclusively managed at the two specialist cancer centers where notification procedures are of high quality.
In conclusion, BC mortality in Estonia has been in decline since 2000 and considerable improvement in BC survival was observed over the study period. Women under 60 years of age benefited most from both earlier diagnosis and treatment advances of locally/regionally spread cancers. However, there is still much room for improvement, as the survival gap with more developed countries persists. It is clear that further increase in survival could be achieved and a number of premature BC deaths avoided by facilitating early diagnosis in all age groups, particularly among women over 60 years of age. At the same time, investigations should continue to clarify the underlying mechanisms of the stage-specific survival deficit in Estonia. Prompt access to diagnosis and treatment, strict adherence to guidelines and adjustment of these to local needs and possibilities, choosing adequate treatment options for older patients, including best supportive care, and continuous adoption of new technologies for diagnosis and therapy would probably help to contribute to further progress. The results of this study are likely to be relevant for other countries of health care transition of Eastern Europe.
Acknowledgments
The authors thank Dr Margit Mägi and Mrs Pille Härmaorg from the Estonian Cancer Registry for providing the data, and Dr Saima Mae for technical help.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The study was supported by Estonian Science Foundation (grant no ETF8881), Estonian Ministry of Education and Research (SF0940026s07) and Estonian Research Council (IUT5-1).
References
- Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, et al. EUROCARE-3 summary: Cancer survival in Europe at the end of the 20th century. Ann Oncol 2003; 14(Suppl 5):v128–49.
- Gondos A, Bray F, Hakulinen T, Brenner H. Trends in cancer survival in 11 European populations from 1990 to 2009: A model-based analysis. Ann Oncol 2009;20:564–73.
- Sant M, Allemani C, Capocaccia R, Hakulinen T, Aareleid T, Coebergh JW, et al. Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int J Cancer 2003;106:416–22.
- Rahu M. Estonia. In: Parkin DM, Muir CS, Whelan SL, Gao Y-T, Ferlay J, Powell J, editors. Cancer incidence in five continents, Vol VII. Lyon: International Agency for Research on Cancer; 1997. p. 569–73.
- World Health Organization, mortality database [Internet]. [updated 2012 Nov 26; cited 2013 Feb 4]. Available from: http://www.who.int/whosis/mort/download/en/index.html.
- Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. Bethesda, MD: End Results Evaluation Section, National Cancer Institute; 1959.
- Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up-to-date’ cancer survival data: Theory, empirical evaluation, computational realisation and applications. Eur J Cancer 2004;40:326–35.
- Talbäck M, Dickman PW. Predicting the survival of cancer patients recently diagnosed in Sweden and an evaluation of predictions published in 2004. Acta Oncol 2012;51:17–27.
- Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer 2004;40:2494–501.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Dickman PW, Coviello E, Hills M. Estimating and modelling relative survival [Internet] [cited 2013 Jan 11]. Available from: www.pauldickman.com/survival/strs.pdf.
- Aareleid T, Brenner H. Trends in cancer patient survival in Estonia before and after the transition from a Soviet Republic to an open market economy. Int J Cancer 2002; 102:45–50.
- Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JWW. Recent trends of cancer in Europe: A combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer 2008;44:1345–89.
- Finnish Cancer Registry. Cancer in Finland 2008 and 2009. Helsinki: Cancer Society of Finland Publication No 84; 2011.
- Holleczek B, Brenner H. Trends of population-based breast cancer survival in Germany and the US: Decreasing discrepancies, but persistent survival gap of elderly patients in Germany. BMC Cancer 2012;12:317.
- Aasmaa A. Viis aastat rinnavähi sõeluuringuid Eestis: organiseeritud sõeluuringuprogrammi kujunemine ja esimesed tulemused [In Estonian]. Eesti Arst 2007;86:804–8.
- Giordano L, von KL, Tomatis M, Majek O, de WC, Lancucki L, et al. Mammographic screening programmes in Europe: Organization, coverage and participation. J Med Screen 2012;19(Suppl 1):72–82.
- Allemani C, Sant M, Weir HK, Richardson LC, Baili P, Storm H, et al. Breast cancer survival in the US and Europe: A CONCORD high-resolution study. Int J Cancer 2013; 132:1170–81.
- Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med 1981; 304:10–5.
- McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 2012;30:4493–500.
- Ruiterkamp J, Ernst MF, de ML, van der Heiden-van der Loo, Bastiaannet E, van de Poll-Franse LV, et al. Improved survival of patients with primary distant metastatic breast cancer in the period of 1995–2008. A nationwide population-based study in the Netherlands. Breast Cancer Res Treat 2011; 128:495–503.
- Ali AM, Greenberg D, Wishart GC, Pharoah P. Patient and tumour characteristics, management, and age-specific survival in women with breast cancer in the East of England. Br J Cancer 2011;104:564–70.
- Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2009;94:1209–15.
- Väljaots K, Tekkel M, Innos K. Viivitus esmasel arsti poole pöördumisel ja selle põhjused rinnavähipatsientidel Eestis [In Estonian]. Eesti Arst 2012;91:121–7.
- Dickman PW, Hakulinen T, Luostarinen T, Pukkala E, Sankila R, Soderman B, et al. Survival of cancer patients in Finland 1955–1994. Acta Oncol 1999;38(Suppl 12):1–103.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- The Council of the European Union. Council Recommendation of December 2003 on cancer screening. The Council of the European Union 2003/878/EC. OJ Eur Union 2003; L327/34–8.
