Abstract
Purpose. To investigate the robustness of single vocal cord intensity modulated radiation therapy (IMRT) treatment plans for set-up errors, respiration, and deformation. Material and methods. Four-dimensional computed tomography (4D-CT) scans of 10 early glottic carcinoma patients, previously treated with conventional techniques, were used in this simulation study. For each patient a pre-treatment 4D-CT was used for IMRT planning, generating a reference dose distribution. Prescribed PTV dose was 66 Gy. The impact of systematic set-up errors was simulated by applying shifts of ± 2 mm to the planning CT scans, followed by dose re-calculation with original beam segments, MUs, etc. Effects of respiration and deformation were determined utilizing extreme inhale and exhale CT scans, and repeat scans acquired after 22 Gy, 44 Gy, and 66 Gy, respectively. All doses were calculated using Monte Carlo dose simulations. Results. Considering all investigated geometrical perturbations, reductions in the clinical target volume (CTV) V95%, D98%, D2%, and generalized equivalent uniform dose (gEUD) were limited to 1.2 ± 2.2%, 2.4 ± 2.9%, 0.2 ± 1.8%, and 0.6 ± 1.1 Gy, respectively. The near minimum dose, D98%, was always higher than 89%, and gEUD always remained higher than 66 Gy. Planned contra-lateral (CL) vocal cord DMean, gEUD, and V40 Gy were 38.2 ± 6.0 Gy, 43.4 ± 5.6 Gy, and 42.7 ± 14.9%. With perturbations these values changed by −0.1 ± 4.3 Gy, 0.1 ± 4.0 Gy, and −1.0 ± 9.6%, respectively. Conclusions. On average, CTV dose reductions due to geometrical perturbations were very low, and sparing of the CL vocal cord was maintained. In a few observations (6 of 103 simulated situations), the near-minimum CTV-dose was around 90%, requiring attention in deciding on a future clinical protocol.
Intensity modulated radiation therapy (IMRT) is being increasingly investigated for the management of early stage squamous cell carcinoma of the larynx [Citation1−6]. IMRT produces a conformal dose distribution around the tumor; therefore the surrounding organs at risk (OAR) can be spared more than what could be achieved with conventional radiation therapy (RT) techniques. Limiting the dose to OAR has the potential of reducing RT-related toxicity, such as, vocal dysfunction, dysphagia, trismus, arytenoid edema, carotid arteries injuries, and increased risk of strokes [Citation1,Citation2,Citation7−12]. IMRT appears not widespread as the standard technique for stage T1 laryngeal cancer, and whenever it is applied, both vocal cords are included in the treatment volume [Citation1,Citation2]. The reason for this forbearance in the implementation of IMRT to this specific tumor site, compared to other head-and-neck tumor sites, may be the good overall survival currently achieved [Citation6]. Nevertheless, reducing the dose to normal tissue potentially reduces long-term sequelae and improves the quality of life (QoL), which is important for this category of patients with such a long-term survival. Additionally, limiting the dose to the OARs, potentially yields another treatment option for patients with re-current tumors; re-irradiation. The use of IMRT and small target volumes could potentially also allow shorter, more hypo-fractionated schedules. Safety margins [clinical target volume-planning target volume (CTV-PTV)] are used to ensure that the CTV receives adequate coverage during the multi-fraction course of treatment.
IMRT plans usually contain high dose gradients to spare OAR adjacent to target volumes, putting an enhanced stress on selection of appropriate margins. This is particularly important as it was shown that a small position variation does not necessarily mean a correspondingly small dose variation and vice versa in the case of head-and-neck RT because of tissue inhomogeneities and body contour variations with respect to the beams [Citation13]. IMRT utilizes three-dimensional (3D) anatomic information extracted from computed tomography (CT) scans acquired prior to treatment. Nonetheless, several groups have reported anatomic changes occurring throughout fractionated external beam radiotherapy [Citation14,Citation15]. The CTV-PTV margins have to account for potential anatomical changes during the course of treatment, e.g. caused by tumor regression or weight loss. In single vocal cord IMRT, applied CTV-PTV margins also have to compensate for respiratory tumor motion [Citation3,Citation5]. Recently, it has been shown that using adaptive radiotherapy (ART) techniques has potential advantages in preventing many of the above mentioned concerns when treating head-and-neck tumors with IMRT [Citation16,Citation17].
At Erasmus Medical Center in Rotterdam we have developed a highly focused image-guided IMRT technique to only irradiate the involved vocal cord in early glottic cancer. This was allowed by recent technological advances in image acquisition with 4D-CT, image-guided position verification with cone beam CT, and the clinical availability of a treatment planning system (TPS) with Monte Carlo dose calculations [Citation3–6]. With our technique, not only the distant OARs are spared but also the contra-lateral (CL) vocal cord that lies in close vicinity to the irradiated vocal cord [Citation5]. Single vocal cord irradiation (SVCI) with IMRT involves the use of small treatment fields for a tumor surrounded partially by air, complicating selection of appropriate CTV-PTV margins. For the treatment plans generated in [Citation5] (used as reference in this study, see below), an isotropic CTV-PTV margin of 2 mm was used. The current study was conducted to test the robustness of these IMRT plans against different geometrical uncertainties. The uncertainties evaluated were; set-up errors, respiratory movements, and inter-fraction anatomical variations (deformation).
Material and methods
Patients and generation of reference IMRT dose distributions
Ten patients previously treated for early glottic carcinoma (T1aN0M0) with conventional techniques (parallel opposed wedged fields, 6MV photons, 66Gy in 33 fractions) were retrospectively included in this study. The involved cases and generation of the reference IMRT dose distributions as used in this study have been described in detail in [Citation5]. Here a brief summary is provided. Each patient had 4D-CT scans acquired at different time points; before treatment, after 22 Gy, 44 Gy, and 66 Gy. Patient 6 was enrolled in the study after the start of the treatment and only 4D-CT scans after 22 Gy were available. These 4D-CT scans were used to reconstruct eight breathing phase resolved 3D-CT scans (or frames) [Citation3]. The CT resolution was 1 × 1× 1 mm3. The area of the airgap at the level of the vocal cords was measured on the different frames and a middle 3D-CT scan/frame of the 4D-CT data set was determined in each case. The pre-treatment middle 3D-CT scan representing the local anatomy on (or closest to) its average respiratory position was used for planning. Using this scan for planning, systematic error could be reduced to nearly zero and respiratory motion contribution to the margins will stem from purely random errors [Citation18]. The CTV was defined as the whole affected vocal cord. To construct a CTV-PTV safety margin for planning a standard margin formula: M = 2.5Σtot + 1.64σtot − 1.64σp where 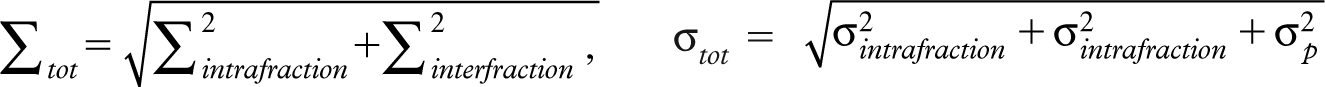 and σp is the SD describing the penumbra width (σp ≈ 0.4 cm in SVCI plans) was used. We included uncertainties originated from intra-fraction respiration [Citation3], residual inter-fraction set-up when employing on-line setup corrections using daily cone beam CT as explained in our previously published work [Citation4]. Intra-fraction set up uncertainties was estimated to be equal to the inter-fraction uncertainties as a reasonable estimate. Using this procedure, margins of 0.5, 1.7, and 0.8 mm in the medio-lateral, cranio-caudal, and antero-posterior directions were calculated. Given the 1 mm3 resolution of the CT scan, practical margins of 2 mm in all directions were used. IMRT plans were generated on these planning scans and the corresponding dose distributions were the reference distributions as used in this study. A class solution with five beam angles was employed [Citation5]. The prescribed dose to the CTV was always 66 Gy given in 33 fractions (six weekly fractions) using 6-MV photon beams. The IMRT treatment plans were generated with the Monaco TPS, version 1.00 (Elekta-CMS) that uses a Monte Carlo dose calculation engine.
and σp is the SD describing the penumbra width (σp ≈ 0.4 cm in SVCI plans) was used. We included uncertainties originated from intra-fraction respiration [Citation3], residual inter-fraction set-up when employing on-line setup corrections using daily cone beam CT as explained in our previously published work [Citation4]. Intra-fraction set up uncertainties was estimated to be equal to the inter-fraction uncertainties as a reasonable estimate. Using this procedure, margins of 0.5, 1.7, and 0.8 mm in the medio-lateral, cranio-caudal, and antero-posterior directions were calculated. Given the 1 mm3 resolution of the CT scan, practical margins of 2 mm in all directions were used. IMRT plans were generated on these planning scans and the corresponding dose distributions were the reference distributions as used in this study. A class solution with five beam angles was employed [Citation5]. The prescribed dose to the CTV was always 66 Gy given in 33 fractions (six weekly fractions) using 6-MV photon beams. The IMRT treatment plans were generated with the Monaco TPS, version 1.00 (Elekta-CMS) that uses a Monte Carlo dose calculation engine.
Simulation of set-up errors
The effects of set-up errors on CTV dose coverage and on the dose received by the CL vocal cord were assessed by re-computing the dose distribution for six shifts of the iso-center on the planning CT scan along the three principal axes (+ and −). Dose re-calculation was performed using the linac parameters (segments, MUs, etc.) as established for generation of the reference dose distribution. In a previous work [Citation4] we have presented a daily on-line positioning procedure for single vocal cord irradiation. We found that the residual positioning errors after employing this on-line 3D patient setup correction procedure were below a millimeter. Nevertheless, in this present study shifts of ± 2 mm were simulated as an upper bound estimate of possible systematic set-up errors in clinical settings. As patient 6 had no pre-treatment 4D-CT, the described analyses were done with the scan acquired after 22 Gy.
Simulation of respiratory motion
For each patient, the extreme inhale and extreme exhale CT scans, reconstructed from the pre-treatment 4D-CT scan, were used for the simulations. The manually delineated planning scan was exported to the Atlas-Based Auto-segmentation (ABAS) software (Elekta/CMS software) to serve as a patient specific atlas [Citation19] for delineation of the two extreme breathing phases CT scans. ABAS delineations were edited by an experienced observer (author 3) [Citation20], therefore, delineation inaccuracies from ABAS were removed in order to avoid increase in delineation uncertainty. Again, dose calculations for these extreme breathing phases were based on the original linac parameters. As patient 6 had no pre-treatment 4D-CT, the described analyses were done with the scan acquired after 22 Gy.
Simulation of deformations and volumetric changes
The impact of deformations and volumetric changes was simulated utilizing repeat 4D-CT scans that were acquired at 22 Gy (eight patients), 44 Gy (six patients) and at the end of the treatment 66 Gy (nine patients). In each repeat 4D-CT, the reconstruction with the anatomy (vocal cords) on its average position was determined and used for this analysis. Delineation of these middle CT scans was performed as described above. To simulate the treatment positioning, rigid registration of the planning CT scan with each repeat CT scan was performed. The coordinates of three well-defined anatomical points; the front node of the thyroid cartilage and the two arytenoids were determined on both CT scans. The registration result of the center of mass (COM) of these three points was then used to determine the shifts applied to the iso-center of the repeat scan, followed by calculation of the corresponding dose distribution. The applied registration method resembles our cone beam CT based, on-line setup verification and correction protocol [Citation4].
Dose calculation and plan evaluation
All dose calculations in this study were done in the Monaco treatment planning system (Elekta/CMS software), using the integrated Monte Carlo-based dose calculation algorithm. In total 103 plans were re-calculated to simulate the uncertainties described above. In all cases, re-calculated dose distributions were compared with the corresponding reference distribution as designed for the planning CT scan. In all cases, the CTV was used to evaluate tumor dose delivery; the PTV was only a geometrical tool to generate a treatment plan. Apart from the CTV, dose in the CL vocal cord was evaluated in detail. For the CTVs, the volume that received dose ≥ 95% of the prescribed dose, V95%, the dose received by 98% of the structure, D98%,(near-minimum dose), the dose received by the hottest 2% of the volume, D2%, and the generalized equivalent uniform dose, gEUD, were calculated. The latter was computed using:
where vi represents the i’th partial volume receiving dose Di in Gy and a is a unit-less model parameter that has a structure specific value that describes the volume effect [Citation21]. As a decrease to a large negative number the gEUD approaches the minimal dose (used for tumors). Similarly as a increases to a large positive number gEUD approaches the maximal dose (serial organs). For a = 0, gEUD is equal to the geometric mean dose and if a = 1 the gEUD is equal to the arithmetic mean dose. In this work for the CTV (a = −13) [Citation22]. For the CL vocal cord, the mean dose, DMean, gEUD (a = 2.2) [Citation10], and the percentage of the volume that received 40 Gy or more, V40 Gy, were assessed. The parameter V40 Gy was reported as it was recommended by the quantitative analysis of normal tissue effects in the clinic (QUANTEC) [Citation10] to limit the mean non-involved larynx dose to 40 Gy or less.
Results
CTV-delineation
Population mean CTV volume was 0.5 ± 0.1 cm3, ranging from 0.2–0.8 cm3. A small repeat study pointed at an intra-observer uncertainty of ± 0.08 cm3 in vocal cord delineation. Delineated CT volumes in the various CT scans (pre-treatment and repeat scans) used in this study were analyzed. It was found that CTV delineations for the different time points were consistent (see Electronic Figure 1, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.812793).
Impact of geometric perturbations on CTV dose distributions
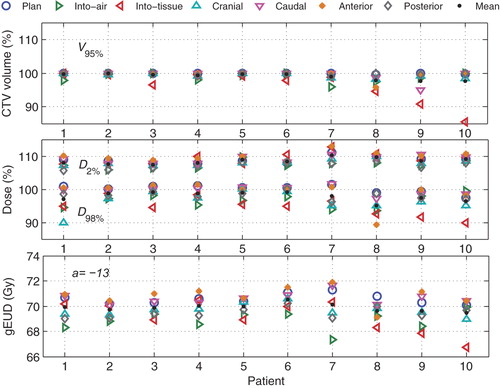
Averaged over all patients and simulated systematic shifts, changes in the CTV V95%, D98%, D2%, and gEUD compared to the reference dose distributions were 1.1 ± 2.4%, 2.7 ± 2.9%, 0.4 ± 1.4%, and 0.8 ± 1.0 Gy, respectively (). The largest changes were generally observed for 'into-tissue’ lateral shifts. Even for patient 10, with lowest V95% and D98%, the gEUD value remained above 66 Gy with D98% = 89%.
Figure 1. Variations in CTV dose parameters with shifting the iso-center by 2 mm in different directions. In the upper panel the percentage of the CTV volume receiving dose of ≥ 95% of the prescribed dose is shown. The dose received by 98% of the CTV volume, and the dose percentage received by the hottest 2% of the CTV volumes are presented in the middle panel. In the lower panel the CTV generalized equivalent uniform dose for each simulated situation is shown.
The impact of respiration phase on CTV dose parameters was relatively small. Averaged over the 10 patients and considering both inhale and exhale scans, V95%, D98%, D2%, and the (gEUD) changed by 1.0 ± 2.0%, 1.9 ± 2.8%, 0.7 ± 1.0%, and 0.6 ± 0.7 Gy, respectively (see Electronic Figure 2, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.812793).
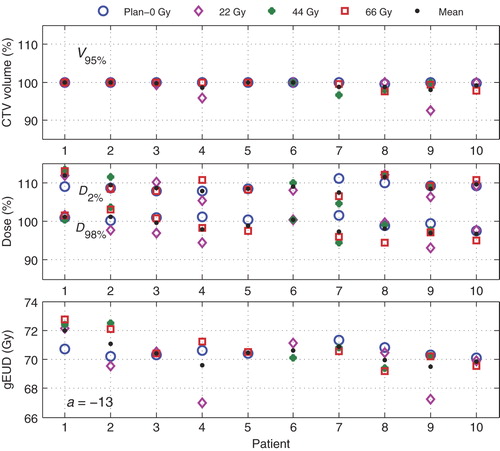
shows the impact of deformations and other volumetric changes during the course of fractionated treatment. Apart from the 22 Gy scans of patients 4 and 9, all deviations from the 0 Gy planning scan are minor. No clear explanation for the larger deviations is available as there is no indication of a change in delineated CTV. However, in all cases, the gEUD values were larger than 67 Gy, and D98% values were never lower than 93%. For all 22 Gy, 44 Gy and 66 Gy scans together, V95%, D98%, D2%, and the ( gEUD) changed by 1.0 ± 1.9%, 1.9 ± 3.0%, −0.5 ± 2.8%, and 0.1 ± 1.5 Gy on average.
Figure 2. Variations in some CTV dose parameters with preceding treatment time shown at different time points 0 Gy, 22 Gy, 44 Gy, and 66 Gy for all patients.
Considering all investigated geometrical perturbations, the overall reductions in the CTV V95%, D98%, D2%, and gEUD were 1.2 ± 2.2%, 2.4 ± 2.9%, 0.2 ± 1.8%, and 0.6 ± 1.1 Gy, respectively.
Impact of geometric perturbations on CL vocal cord sparing
The population averages of the planned DMean, gEUD, and V40 Gy of the CL vocal cord (presented with circles in and (Electronic Figure 3, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.812793) are 38.2 ± 6.0 Gy, 43.4 ± 5.6 Gy, and 42.7 ± 14.9%, respectively. As expected, the shift analyses in show largest increase of the CL vocal cord dose with iso-centric shifts towards airgap and the contrary with shifts away from the airgap. Considering all systematic shifts in all patients, DMean, gEUD, and V40 Gy, changed compared to planning by −0.4 ± 4.9 Gy, −0.2 ± 4.7 Gy, and −1.2 ± 10.7%, respectively. Clearly, the large gain relative to a conventional RT technique (DMean = 66 Gy, V40 Gy = 100%) is maintained. Even for the shifts giving highest parameters, there is still a considerable gain in terms of sparing.
Figure 3. Variations in the mean dose (DMean), (gEUD) and (V40 Gy) received by the CL vocal cord with iso-centers shifts of 2 mm in the specified directions.
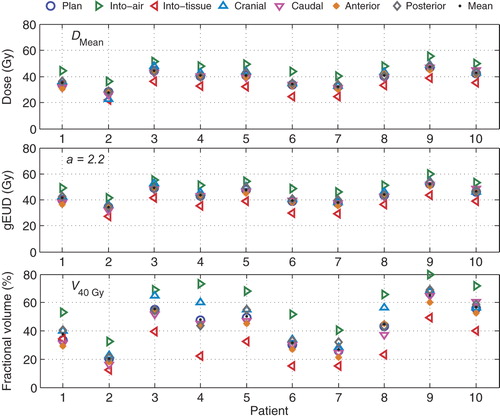
Figure 4. Variations in the mean dose (DMean), (gEUD) and (V40 Gy) received by the CL vocal cord with preceding treatment time shown at different time points 0 Gy, 22 Gy, 44 Gy, and 66 Gy for all patients.
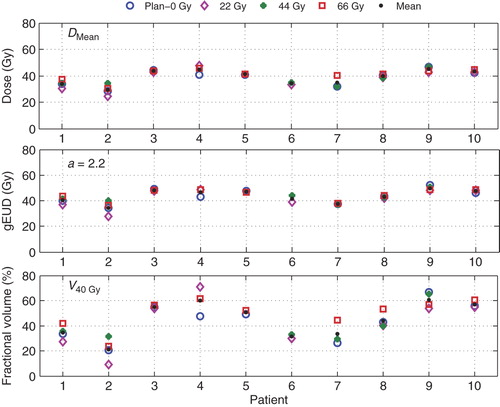
As for the CTV, change in respiration phase resulted in the smallest changes in the CL vocal cord dose parameters. Changes in respiration phase resulted in 1.2 ± 2.3 Gy, 1.2 ± 2.1 Gy, and 1.4 ± 5.1% changes in DMean, gEUD, and V40 Gy, respectively. For the 22 Gy, 44 Gy and 66 Gy scans (), these parameters changed by −0.8 ± 3.6 Gy, −0.1 ± 3.2 Gy, and −2.5 ± 9.3%, respectively (see Electronic Figure 3, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.812793).
Considering all investigated geometrical perturbations, the overall changes in the CL vocal cord DMean, gEUD, and V40 Gy were −0.1 ± 4.3 Gy, 0.1 ± 4.0 Gy, and −1.0 ± 9.6%, respectively.
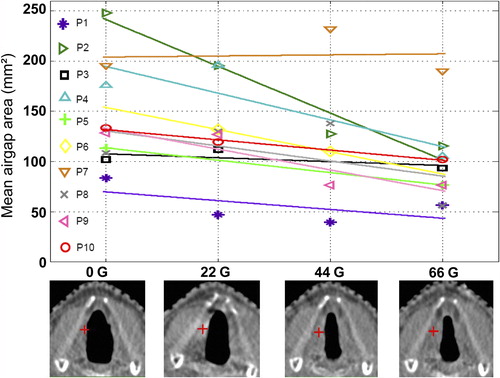
In eight of nine patients with a 0 Gy CT-scan available, V40 Gy after 66 Gy is higher than at planning (). For this reason, we studied the airgap between the vocal cords as a function of delivered dose (). For the 10 patients, when plotting the mean airgap area as a function of the dose, the average of the slopes of the regression lines was negative (p = 0.004), indicating that there is an overall decrease in the airgap areas with progressing treatment time. This could possibly be explained by the incidence of arytenoid edema or synechia in the anterior commissure that are known acute RT side effects.
Figure 5. Upper panel: For each patient, the airgap area between the vocal cords, averaged over the various breathing phases (various axial CT-slices), as a function of delivered dose is presented. The lines are results of regression analyses, with on average, a statistically significant negative slope (see text). Lower panel: axial CT slices for patient 2 at the level of the iso-center (indicated by a red +) for doses 0 Gy, 22 Gy, 44 Gy, and 66 Gy.
Discussion
We have been the first group to propose a complete systematic image-guided procedure for the delivery of highly focused IMRT plans for single vocal cord irradiation [Citation3–6]. The design of CTV-PTV margins for small tumors, partially surrounded by air, is non-trivial. Based on assessed geometrical uncertainties, in a previous study [Citation5], we decided on the use of a 2 mm margin for SVCI. The present analysis was performed to dosimetrically test the robustness of the IMRT plans generated with this 2 mm margin [Citation5]. We used the PTV for planning and the CTV to evaluate target dose for investigated geometrical variations. Dose was always recalculated using beam angles, segments, MUs, etc. as established during treatment plan generation. Dose distributions were (re-)calculated with Monte Carlo simulation, taking into account all real internal inhomogeneities and surface curvatures.
Three possible sources of geometrical variation, i.e. set-up errors, respiratory movements, and inter-fraction volumetric changes and deformation were investigated. Planned IMRT dose distributions were compared with re-calculated dose distributions for the perturbed geometries. It was found that the good CTV dose coverage and the CL vocal cord sparing achieved in the static reference plans were largely maintained under the influence of treatment simulated geometrical variations. For the CTV, coverage (V95%), near-minimum dose (D98%), and gEUD were on average reduced by 1.2%, 2.4% and 0.6 Gy, respectively. In a few instances, the near-minimum doses, D98%, in perturbed geometries (see ) were around 90% of the prescribed dose, instead of 95%. These were exceptions, and it should also be taken in mind that in the shift analyses, for each individual patient, this only occurred at maximum in 1/6 of the simulated shifts. Moreover, with the proposed on-line protocol in place, 2 mm shifts are unlikely to occur [Citation4], and it is even less likely that they are systematic, i.e. repeating each fraction. In the repeat CT analyses, a lower D98% in a patient repeat scan was always accompanied by higher values at different time points ().
With the current, conventional treatment technique, local failure because of tumor miss is virtually impossible. Based on our results obtained so far ([Citation3–6], this paper), we believe that with daily on-line CBCT-based re-positioning, probability of local failure with our planned IMRT dose distributions will also be very low. However, given the discussion above, there may be some risk that it not as low as for the conventional RT technique.
Gowda et al. [Citation23] reported an excellent five-years local control of 93% following treatment with 50–52.5 Gy in 16 fractions in an overall treatment time (OTT) of 21–26 days. In our current ideas for SVCI, a total dose of 58.1 Gy may be delivered in 16 fractions, 5 fractions per week, yielding a higher total dose than Gowda et al., and an equivalent dose in 2 Gy fractions (EQD2 Gy) of 66 Gy as in our current treatment approach, but in a shorter overall treatment time. A default 3 mm CTV-PTV margin is considered for single vocal cord IMRT (increase by 1 mm). A check on robustness of generated plan against potential anatomy shifts during treatment as described above can be performed for each individual patient prior to the start of treatment. Such a procedure may be used to verify the initially selected default CTV-PTV margin on a per patient basis and adapt the margins and the plan if needed, also in the context of potential inter-patient variations in plan conformity (see and ).
SVCI with IMRT is an experimental technique that has yet to be clinically proven. It is also important to realize that the analyses presented in this article were conducted using patient data from patients that were treated with conventional RT techniques. The effect of the proposed SVCI procedure is still to be determined. In preparation for the SVCI with IMRT, studies on the dosimetry and dose modeling, especially in the buildup region are being conducted (unpublished observations). In our positioning procedure daily CBCT are used for on-line set-up verification. In the future, these CBCT scans could be used in combination with deformable image registration techniques to detect daily volumetric changes and assess the actual delivered doses [Citation24]. This will aid the assessment of the effects asymmetrical dose distribution on the vocal cords and the surrounding structure. In our IMRT plans we used quadratic overdose constraints to limit the dose to unspecified tissue [Citation5]. Nevertheless, special attention should be paid to the potential enhancement of second primary tumors with the relatively larger volume of healthy tissue exposed to low dose of radiation compared to conventional techniques. van Asselen et al. [Citation25] showed that the amplitude of larynx movements due to swallowing may be > 1 cm, but they also demonstrated that the incidence and duration of swallowing are low for most of the patients. Nonetheless, in a clinical protocol, swallowing during imaging and treatment has to be minimized, e.g. by patient instruction and monitoring.
Conclusions
This simulation study shows that with isotropic margins as small as 2 mm and daily cone beam CT-guided repositioning, CTV dose remained generally high, and large sparing of the CL vocal cord was maintained, when considering realistic intra- and inter-fraction geometric variations. Occasionally observed modest reductions in CTV dose have to be considered when deciding on a clinical protocol.
Electronic Figures 1 to 3
Download PDF (1.2 MB)Declaration of interest: The authors wish to thank Eliana Vasquez Osorio and Hafid Akhiat for their technical support during this work. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rosenthal DI, Fuller CD, Barker Jr. JL, Mason B, Garcia JA, Lewin JS, et al. Simple carotid-sparing intensity-modulated radiotherapy technique and preliminary experience for T1-2 glottic cancer. Int J Radiat Oncol Biol Phys 2010; 77:455–61.
- Chera BS, Amdur RJ, Morris CG, Mendenhall WM. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys 2010;77:1380–5.
- Osman SOS, de Boer HCJ, Heijmen BJM, Levendag PC. Four-dimensional CT analysis of vocal cords mobility for highly focused single vocal cord irradiation. Radiother Oncol 2008;89:19–27.
- Osman SOS, de Boer HCJ, Astreinidou E, Gangsaas A, Heijmen BJM, Levendag PC. On-line cone beam CT image guidance for vocal cord tumor targeting. Radiother Oncol 2009;93:8–13.
- Osman SOS, Astreinidou E, de Boer HCJ, Keskin-Cambay F, Breedveld S, Voet P, et al. IMRT for image-guided single vocal cord irradiation. Int J Radiat Oncol Biol Phys 2012; 82:989–97.
- Levendag PC, Teguh DN, Keskin-Cambay F, Al-Mamgani A, van Rooij P, Astreinidou E, et al. Single vocal cord irradiation: A competitive treatment strategy in early glottic cancer. Radiother Oncol 2011;101:415–9.
- Aref A, Dworkin J, Devi S, Denton L, Fontanesi J. Objective evaluation of the quality of voice following radiation therapy for T1 glottic cancer. Radiother Oncol 1997;45: 149–53.
- Verdonck de Leeuw IM, Keus RB, Hilgers FJM, Koopmans van Beinum FJ, Greven AJ, de Jong JMA, et al. Consequences of voice impairment in daily life for patients following radiotherapy for early glottic cancer: Voice quality, vocal function, and vocal performance. Int J Radiat Oncol Biol Phys 1999;44:1071–8.
- Hočevar-Boltežar I, Žargi M, Strojan P. Risk factors for voice quality after radiotherapy for early glottic cancer. Radiother Oncol 2009;93:524–9.
- Rancati T, Schwarz M, Allen AM, Feng F, Popovtzer A, Mittal B, et al. Radiation dose-volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl 1):S64–9.
- Rovirosa A, Biete A. In relation to the arytenoid edema in the radiotherapy of the early vocal cord cancer: Arytenoid shielding and small size of the field. Radiother Oncol 1997; 45:209–10.
- Dorresteijn LDA, Kappelle AC, Scholz NMJ, Munneke M, Scholma JT, Balm AJM, et al. Increased carotid wall thickening after radiotherapy on the neck. Euro J Cancer 2005;41:1026–30.
- Astreinidou E, Bel A, Raaijmakers CPJ, Terhaard CHJ, Lagendijk JJW. Adequate margins for random setup uncertainties in head-and-neck IMRT. Int J Radiat Oncol Biol Phys 2005;61:938–44.
- Barker JL, Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys 2004;59:960–70.
- Senkus-Konefka E, Naczk E, Borowska I, Badzio A, Jassem J. Changes in lateral dimensions of irradiated volume and their impact on the accuracy of dose delivery during radiotherapy for head and neck cancer. Radiother Oncol 2006;79:304–9.
- Castadot P, Lee JA, Geets X, Grégoire V. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol 2010;20:84–93.
- Schwartz DL, Dong L. Adaptive radiation therapy for head and neck cancer can an old goal evolve into a new standard?. J Oncol 2011; 2011 Article ID 690595.
- Engelsman M, Damen EMF, de Jaeger K, van Ingen KM, Mijnheer BJ. The effect of breathing and set-up errors on the cumulative dose to a lung tumor. Radiother Oncol 2001;60: 95–105.
- Teguh DN, Levendag PC, Voet PWJ, Al-Mamgani A, Han X, Wolf TK, et al. Clinical validation of atlas-based auto-segmentation of multiple target volumes and normal tissue (swallowing/mastication) structures in the head and neck. Int J Radiat Oncol Biol Phys 2011;81:950–7.
- Voet PWJ, Dirkx MLP,Teguh DN, Hoogeman MS, Levendag PC, Heijmen BJM. Does atlas-based autosegmentation of neck levels require subsequent manual contour editing to avoid risk of severe target underdosage?A dosimetric analysis. Radiother Oncol 2011;98:373–7.
- Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys 1997;24:103–10.
- Gay HA, Niemierko A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys Med 2007;23:115–25.
- Gowda RV, Henk JM, Mais KL, Sykes AJ, Swindell R, Slevin NJ. Three weeks radiotherapy for T1 glottic cancer: The Christie and Royal Marsden Hospital Experience. Radiother Oncol 2003;68:105–11.
- Elstrøm UV, Wysocka BA, Muren LP, Petersen JB, Grau C. Daily kV cone-beam CT and deformable image registration as a method for studying dosimetric consequences of anatomic changes in adaptive IMRT of head and neck cancer. Acta Oncol 2010;49:1101–8.
- van Asselen B, Raaijmakers CPJ, Lagendijk JJW, Terhaard CHJ. Intrafraction motions of the larynx during radiotherapy. Int J Radiat Oncol Biol Phys 2003;56:384–90.
