To the Editor,
About 20% of colorectal cancer patients have disseminated disease at the time of diagnosis, and for the vast majority curative treatment is not possible. There are many options to treat the primary tumor in these patients, but it is not known if the survival of the patients is influenced by the treatment of the primary tumor. If the primary tumor is symptomatic, with obstruction, perforation, pain or bleeding, it can be treated by resection, bypass surgery, proximal stoma or stent. If the primary tumor is asymptomatic or gives rise only to mild symptoms the choice is between primary resection followed by chemotherapy or upfront chemotherapy in patients fit enough for these treatments. Arguments for resection of the primary tumor are that symptoms, or the risk for the development of late symptoms, are eliminated and the possibility to improve survival by reducing the tumor burden and eliminate the source of metastases. Arguments against resection are the risk for mortality and morbidity following surgery and that the medical oncological treatment options are delayed by surgery.
There are numerous studies evaluating the effect of resection of the primary tumor on survival in stage IV colorectal cancer. The studies are of different designs, from different time periods and show conflicting results. All studies are retrospective and non-randomized. Three meta-analyses [Citation1–3] have been published during 2008–2010 and all three came to different conclusions. Most studies show a survival benefit for the patients who have had their primary tumor removed. In the meta-analysis by Stillwell et al. [Citation3] the survival gain is calculated to six months. In the studies there are, however, several factors that can bias the results. Resected patients are probably more fit and with less tumor burden than those not resected. Further, in most studies, old and thus less effective medical oncological treatments have been used.
The uncertainty regarding the effect on survival from resection of the primary tumor seemed an important reason to conduct an adequately powered randomized prospective trial. Subsequently, a Swedish trial, the Primarily Generalized Colorectal cancer (PGC-) trial, regarding the treatment strategies for patients with primary metastatic colorectal cancer was designed.
Method
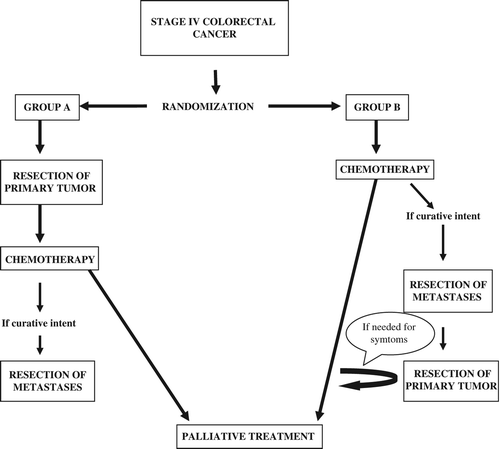
The trial was set up to be a multicenter randomized prospective trial with two treatment arms reflecting the different strategies in patients where there is uncertainty regarding the best strategy ().
Eligible to enter the study were patients with disseminated colorectal cancer at the time for diagnosis and where there was uncertainty regarding the best treatment strategy ().
Table I. Inclusion- and exclusion criteria.
The primary endpoint was overall survival. Secondary endpoints were quality of life, healthcare consumption, complications to treatment, tumor situation at three years for patients treated with curative intent, number of patients not receiving chemotherapy in arm A and number of patients needing any surgical intervention for their primary tumor in arm B.
Power calculation was made according to Collett [Citation4] and gave a sample size a little short of 500 patients. We assumed a difference in survival of 30% as clinically relevant. Power was set to 80% and significance level 5%. Stratification was on center and randomization was done in blocks for each participating center using www.randomization.com.
The study was approved by the Regional ethics committee (M170-07) in Linköping and registered at www.clinicaltrials.gov (NCT 01056809).
To make the study simple and easy for patients and investigators to accept, no extra visits, tests or examinations were required. Apart from quality-of-life forms (EORTC QLQ-C30, CR-29 and EQ5-D) every six months only data normally put into the patients charts and the national quality registry [Citation5] was used. The national quality registry was meant to serve as a log list of not included patients, so investigators did not need to keep separate log lists.
Results
The study started in January 2010 after an invitational letter to all members of the Swedish Society for Colon and Rectal Surgeons. Before and during the study period information about the study was given at numerous meetings among Swedish surgeons and clinical oncologists and also at meetings in Denmark and Norway and at the World Congress of Gastrointestinal Cancer in Barcelona, Spain.
The study gained a lot of interest in Sweden, Norway, Denmark and elsewhere and was considered an important study by many leading surgeons and oncologists. Eventually, however, only 11 Swedish centers accepted to participate and the total number of randomized patients after two years was 15 from five centers. The study was closed in June 2012.
Discussion
It is disturbing that a study considered important in an international perspective and by many potential local investigators ends up with only 11 participating centers. It is even more disturbing that these centers, with a catchment area of more than two million people, only recruit 15 patients in two years. Two similar studies, the ISAAC study (Clinical Trials NCT 01086618) in UK and the SUPER study (Australian New Zealand Clinical Trials Registry ACTRN 12609000680268) in New Zealand and Australia have also been closed due to poor inclusion rates. In Australia/NZ three patients were randomized from four centers during 12 months and in GB 24 patients were randomized from 40 centers during 18 months (pers. comm.). We have identified some factors that may explain the low inclusion rate.
The patient population is smaller than anticipated. In Sweden about 1000 patients are diagnosed yearly with stage IV colorectal cancer, but in reality only a small fraction fits the inclusion/exclusion criteria. Many patients are too old or too sick to be candidates for either surgery or chemotherapy. Even in fit patients many have far too advanced metastatic disease and thus, according to the clinical evaluation in the multidisciplinary team conference (MDT-meeting), has little to gain from resection of the primary tumor. It can be argued that to aim for 500 patients is too optimistic, but we did not want the study to be underpowered and both the ISAAC and the SUPER study reached about the same sample sizes (500 and 400, respectively) in their power calculations.
The expansion of liver surgery is another factor. The indications for conversion- (neo-adjuvant-) chemotherapy are now very wide, since many patients with advanced liver disease are treated with the aim to potentially undergo surgery. However, many of these will never be candidates for liver resection. In order to recruit them in the study, both patients with potentially curable or incurable disease could be included. This has made the inclusion/exclusion criteria indistinct and has caused some confusion among investigators in the study, though it was repeatedly stressed that it was not a “liver first”-study. Many of the patients were judged to need chemotherapy at once to have a chance to become resectable and have thus not been candidates for the study. This was in fact one the most common causes for exclusion in the trial. When the limits of meaningful liver surgery can be defined, the prerequisite of a study may increase [Citation6].
Another reason for poor inclusion is of course the opinion of the surgeon or the oncologist in charge of the patient. The inclusion criterion states that the responsible doctor has to be uncertain regarding the best treatment strategy, moreover, this should be agreed to in a MDT-meeting. During the planning phase of the study, it was estimated that a rather small fraction of the patients should be judged to have a clear best strategy and that uncertainty was present in a substantial proportion. In Sweden, however, many doctors consider the new chemotherapy and antibody combinations and stent treatment in case of obstruction to be so effective and safe [Citation7], that resection should be reserved for those where there is a possibility for cure. In a study by Frago et al. [Citation8] this is questioned. In the study 55 patients with obstruction due to stage IV colorectal cancer were included. Six patients had their primary tumor resected at inclusion because of perforation and 49 were stented. Early and late stent-failure occurred in 23 patients, three patients received supportive care, nine patients were re-stented and 11 patients had surgery among whom six had their primary tumor resected. The majority of the patients were treated with state-of-the-art FOLFOX or FOLFIRI chemotherapy regimes. Median survival in the 12 resected patients was 23.7 months and in the 43 stented patients 4.4 months. A survival advantage for resected patients is also found in the Swedish Rectal Cancer Registry [Citation9], where the median survival is almost a year longer. Even if patient selection can explain some of this difference, doctors have to be aware of these findings not to be prejudiced against resection of the primary tumor. Further, the median survival of two years and more in the most recent medical oncological trials are to a major extent caused by extensive patient selection [Citation10]. In population-based series median survival is about one year [Citation11].
The patients in the study had to choose between two treatment strategies that may not seem entirely equal, yet in our study only a few patients refused to participate. In the SUPER study refusal to participate was the main problem (pers. comm.). It can be questioned if survival is the right primary endpoint, patients might appreciate other aspects of life with incurable cancer such as quality of life. The aim of this study was, however, to find out if resection of the primary tumor prolongs life and, if so, for how long and at what cost in terms of post-operative complications. With this information the patient can make a well-founded decision about his or her future treatment aims.
In conclusion, this is the third randomized study regarding treatment of the primary tumor in stage IV colorectal cancer that fails. The patient population is much smaller than may be anticipated and many doctors have a clear perception of the best treatment strategy. If we want to find out if resection of the primary tumor improves survival or have other advantages and, if so, justifies the morbidity and mortality following surgery, a randomized adequately powered study is necessary. Other study designs have been criticized for bias, and cannot be expected to reliably answer the posed questions and national quality registries can present an overview but several uncertainties will remain. When the limits of liver surgery are established in relation to the efficacy of the medical oncological treatment, a study may be possible if it involves many centers and the above factors are taken into account.
Acknowledgments
Apart from the authors, the following persons were involved in working out the study protocol: Ulf Gunnarsson, Lars Lundell and Per-Olov Nyström, Department of Surgery, Karolinska Institutet, Huddinge, Per Sandström, Department of Surgery and Hans Starkhammar, Department of Oncology, University Hospital, Linköping, Sweden. This study was funded by the Medical Research Council of Southeast Sweden
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Eisenberger A, Whelan RL, Neugut AI. Survival and symptomatic benefit from palliative primary tumor resection in patients with metastatic colorectal cancer: A review. Int J Colorectal Dis 2008;23:559–68.
- Scheer MGW, Sloots CEJ, van der Wilt GJ, Ruers TJM. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann Oncol 2008;19:1829–35.
- Stillwell AP, Buettner PG, Ho Y-H. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 2010;34:797–807.
- Collett D. Modelling survival data in medical research. Boca Raton, Chapman & Hall-CRC; 2003.
- Påhlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjödahl R, et al. The Swedish rectal cancer registry. Br J Surg 2007;94:1285–92.
- Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: Recommendations from an expert panel. Ann Oncol 2009;20:985–92.
- McCahill LE, Yothers G, Sharif S, Petrelli NJ, Lai LL, Bechar N, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: Definitive analysis of NSABP trial C-10. J Clin Oncol 2012;30:3223–8.
- Frago R, Kreisler E, Biondo S, Salazar R, Dominguez J, Escalante E. Outcomes in the management of obstructive unresectable stage IV colorectal cancer. Eur J Surg Oncol 2010;36:1187–94.
- Hosseinali Khani M, Påhlman L, Smedh K. Treatment strategies for patients with stage IV rectal cancer: A report from the Swedish Rectal Cancer Registry. Eur J Cancer 2012; 48:1616–23.
- Glimelius B, Cavalli-Björkman N. Metastatic colorectal cancer: Current treatment and future options for improved survival. Medical approach – present status. Scand J Gastroenterol 2012;47:296–314.
- Sorbye H, Pfeiffer P, Cavalli-Björkman N, Qvortrup C, Holsen MH, Wentzel-Larsen T, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 2009;115:4679–87.