Abstract
Background. Testicular cancer survivors treated with chemotherapy are at increased risk for metabolic syndrome (MetS) and cardiovascular disease (CVD). We explored acute effects of chemotherapy by assessing metabolic factors, abdominal fat volume, hepatic triglyceride content (HTC) and aortic wall stiffness. Material and methods. We studied 19 testicular cancer patients (age 20–54 years) before, at three and nine months after the start of chemotherapy. Blood serum was analyzed for lipids, glucose and insulin. Abdominal visceral and subcutaneous fat volume and aortic pulse wave velocity were assessed by magnetic resonance imaging (MRI) techniques; HTC was measured by proton MR spectroscopy. Results. Three months after start of chemotherapy visceral abdominal fat volume had significantly increased from 202 ± 141 to 237 ± 153 ml (p = 0.009) whereas body mass index and subcutaneous fat volume significantly increased nine months after treatment from 24.4 ± 4.0 to 26.4 ± 4.1 kg/m2 (p = 0.01) and from 556 ± 394 to 668 ± 460 ml (p = 0.002) respectively. Serum total cholesterol, low-density lipoprotein cholesterol and insulin also significantly increased three months after start of treatment from 4.88 ± 1.1 to 5.61 ± 1.50 mmol/l (p = 0.002), 3.31 ± 1.16 to 3.73 ± 1.41 mmol/l (p = 0.02) and 5.7 ± 4.4 to 9.6 ± 6.3 mU/ml (p = 0.03), respectively. Nine months after start of chemotherapy serum lipid and insulin concentrations had returned to baseline. HTC increased in seven of the 19 patients (36.8%) during follow-up. Aortic pulse wave velocity remained unchanged at the three time points measured. Conclusion. Cisplatin-based chemotherapy was associated with acute insulin resistance, dyslipidemia and an immediate increase in abdominal visceral adipose tissue and abdominal subcutaneous adipose tissue in testicular cancer patients. A large prospective cohort study with long follow-up is warranted to characterize the time course and relationship between acutely induced obesity and hypercholesterolemia and the development of metabolic syndrome and CVD years later in individual testicular cancer survivors.
Testicular cancer (TC), the most common form of cancer in young male adults, holds an excellent prognosis with cure achieved in the vast majority of patients since the introduction of cisplatin-based chemotherapy [Citation1]. Long-term testicular cancer survivors treated with chemotherapy are at increased risk for the metabolic syndrome (MetS) a constellation consisting of abdominal obesity, hypertension, insulin resistance and dyslipidemia with reported prevalences ranging between 17% and 41% [Citation2,Citation3]. During cisplatin, bleomycin and etoposide (BEP) combination chemotherapy acute life-threatening vascular events are known to occur, while cured long-term survivors are at increased risk to develop cardiovascular disease (CVD) [Citation4–6]. These observations suggest a causal relationship between chemotherapy and the increased prevalence of MetS and CVD reported in long-term TC survivors. It seems likely that disturbances in glucose and lipid metabolism are induced early during chemotherapy, which may aggravate possible direct effects of chemotherapy on the vasculature, and translate in increased prevalence of MetS and CVD years later. Cisplatin has previously been reported to induce hepatic toxicity [Citation7], whereas chemotherapy using a.o. oxaliplatin, a platinum analogue, has been shown to induce marked non-alcoholic steatohepatitis (NASH). Furthermore, visceral fat mass has been shown to be metabolic active and is identified as a major contributor to adverse effects on glucose and lipid metabolism, and endothelial dysfunction [Citation8,Citation9]. Increase in visceral fat mass, insulin resistance and chronic inflammation, or a combination of these, may contribute to the development of MetS and the CVD risk [Citation10,Citation11].
There is ample preclinical and clinical evidence for deleterious effects of cytotoxic agents on vascular cells. For instance, cisplatin has been shown to inhibit vascular remodeling and regeneration in vitro [Citation12], whereas bleomycin and cisplatin both induced endothelial damage [Citation13]. In TC patients, arterial vascular events and Raynaud's syndrome have been frequently reported as side effects of cisplatin- and bleomycin-based chemotherapy [Citation14]. These findings underscore the importance of early detection of chemotherapy-induced metabolic and vascular damage because development of specific preventive measures may contribute to improved health in long-term TC survivors.
Validated magnetic resonance imaging (MRI) provides an accurate assessment of abdominal adipose tissue. Proton magnetic resonance spectroscopy (1H-MRS) allows assessment of changes in hepatic triglyceride content (HTC) reflecting fattening of the liver which may occur as a non-specific response of the liver to toxic insults [Citation15]. 1H-MRS has also been shown to offer a suitable technique to measure acute increases in liver triglyceride, i.e. as a consequence of high calorie intake [Citation16]. Indeed, MetS has been suggested to be a predictor of NASH or vice versa [Citation17], and acutely induced liver changes which can be captured by 1H-MRS could be an early sign of MetS which will become clinically manifest years thereafter [Citation18]. The use of MR-based assessment of pulse wave velocity (PWV) of the aorta also enables to assess vascular stiffness, which has been shown to be related to cardiovascular morbidity and mortality [Citation19,Citation20].
With increasing numbers of cancer survivors and greater recognition of MetS, CVD and other late effects of chemotherapy, investigation of early determinants of sequelae of cancer treatment becomes increasingly important. We explored if changes in HTC, aortic PWV, and abdominal visceral and subcutaneous fat volume could be measured in testicular cancer patients undergoing curative treatment by using validated 1H-MRS and MRI techniques.
Material and methods
Between 2007 and 2009 patients with metastatic TC scheduled for first-line curative cisplatin-based combination chemotherapy in the Department of Clinical Oncology were included. The study was approved by the Medical Ethical Committee and all patients gave written informed consent to participate in the trial. Exclusion criteria were co-morbidities, including CVD, diabetes mellitus and hepatic diseases. Of the 40 patients asked to participate, 21 (52.5%) were not able to participate; five (12.5%) for logistic reasons and 16 (40.0%) had unwillingness to undergo frequent blood drawings during chemotherapy (n = 13) or MRIs (n = 3). Nineteen (47.5%) consecutive patients with an otherwise unremarkable medical history were included.
Patients received three or four cycles of BEP-combination chemotherapy repeated every three weeks. Each cycle consisted of intravenously administered etoposide (100 mg/m2 over 1 hour, days 1–5), cisplatin (20 mg/m2 over 4 hours, days 1–5), and bleomycin (30 IUSP over 30 minutes) at days 2, 8, and 15. One patient received additional paclitaxel (175 mg/m2) on day 1 of each of his four chemotherapy cycles. Another patient received two additional courses of vinblastine, ifosfamide and cisplatin (VIP) after his four courses of BEP. The anti-emetic regimen consisted of intravenously administered granisetron (1 mg, days 1–5) and high-dose dexamethasone (10 mg, days 1–5), which was tapered according to the schedule oral dexamethasone two times 3 mg (days 6–7) and two times 1.5 mg (days 8–9) in most patients.
Patients underwent three measurements, i.e. before, and three and nine months after the start of chemotherapy. All patients filled in a form concerning medical history, drug use, life style and intoxications. Blood pressure, heart rate, body weight and height were recorded and body mass index (BMI, weight/height2) was calculated. Overweight was defined as a BMI > 25 kg/m2 and obesity was defined as a BMI > 30 kg/m2. After an overnight fast, MRI measurements were performed and blood samples collected for assessments of lipid profile [total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides], and glucose, insulin and glycosylated hemoglobin (HbA1c) using standard assays. In addition, serum levels of aspartate transaminase (ASAT), alanine transaminase (ALAT), gamma-glutamyl transferase (γ-GT), alkaline phosphatase (AP), lactate dehydrogenase (LDH) and creatinine were measured. Hormone levels, i.e. luteinizing hormone (LH), follicle stimulation hormone (FSH), testosterone, estradiol and sex hormone binding globulin (SHBG) were also measured. All analytes were measured using routine laboratory assays at the Central Laboratories for Clinical Chemistry. Insulin resistance was quantified using the homeostatic model assessment (HOMA) [insulin (mU/l)*glucose (mmol/l)]/22.5 [Citation21], and insulin resistance was considered above the 75th percentile (> 3.30) [Citation22]. Renal function was calculated using the Cockcroft formula.
Magnetic resonance measurements
All MRI measurements were performed in the morning after an overnight fast using a 1.5-T whole-body MRI scanner (Gyroscan ACS/NT15; Philips, Best, The Netherlands). 1H-MRS of the liver was performed with an 8-ml voxel positioned in the liver, avoiding gross vascular structures and adipose tissue depots. The 12th thoracic vertebra served as a landmark to ensure the same position of the voxel during all measurements. Spectra were obtained with an echo time of 26 ms and a repetition time of 3000 ms. A total of 64 data points were collected using a 1000-Hz spectral width with and without water suppression. Spectroscopic data were fitted using validated software (jMRUI version 2.2; developed by A. van den Boogaart, Katholieke Universiteit Leuven, Leuven, Belgium). HTC was calculated as the ratio of the signal amplitude of TGs over the signal amplitude of water × 100%. HTC measurement with respiratory navigator has a coefficient of variation of 17.9% [Citation23].
Based on other reported data, we defined a HTC below 6% as normal [Citation24]. Abdominal visceral and subcutaneous fat volumes were assessed by imaging three consecutive transverse slices during one breath hold, with the middle image at a level just above the fifth lumbar vertebra. The volumes of the visceral and subcutaneous fat depots of all slices were calculated by converting the number of pixels to square centimeters multiplied by the slice thickness. The total volume of the fat depots was calculated by summing fat volumes of all three slices.
Aortic PWV was measured as followed. A retrospective ECG-gated gradient-echo pulse sequence with velocity encoding was applied to measure through-plane flow at two predefined positions in the mid-ascending aorta and abdominal aorta just above the bifurcation. Images were analyzed with MASS/FLOW and aortic PWV was calculated as Δx/Δt, where Δx is the path length between the two measurement sites and Δt is the time delay between the arrivals of the foot of the pulse wave at the respective measurement sites.
For various different logistic reasons including claustrophobia (n = 1) and withdrawal of informed consent (n = 2), chemotherapy-related death due to an acute cerebral vascular event, and 1H-MRS measurements hardware problems (n = 4), hepatic TG content could not be measured before chemotherapy in three patients, could not be performed at time point three months in seven patients and not at time point nine months in another patient.
Aortic PWV data was available for 16, 14, and 13 patients at baseline, second and third measurement, respectively. The reasons for missing measurements were largely similar as for the hepatic triglyceride measurements, but only three of the 46 aortic PWV measurements failed.
Statistical analysis
In the text of the result section, data are provided as mean and SD or as median and range.
To allow the use of all available data, including incomplete profiles, repeatedly measured parameters were compared with a mixed model analysis of variance with fixed factors time and random factor subject. The analysis of (co)variance and Pearson correlations were performed on log transformed data, to correct for the log-normal distribution of the data. The variables that could not be analyzed parametrically because of non-normal distribution were analyzed with a non-parametric Friedman and Wilcoxon test. The following contrasts were calculated: before chemo versus three months measurements and versus nine months and when applicable the three months measurement was compared with the nine months measurement. Estimates of differences of the different contrasts and a back transformed estimate of the difference in percentage for log transformed parameters, 95% confidence intervals (in percentage for log-transformed parameters) and least square means (geometric means for log transformed parameters), and the p-value of the contrasts are provided in the tables. All calculations were performed using SAS for windows V9.1.3 (SAS Institute, Inc., Cary, NC, USA). A Bonferroni-Holm adjustment for multiple testing was made per set of variables for comparison of clinical characteristics and laboratory values reported in , and and for the MRI results in .
Table I. Baseline characteristics of all 19 patients.
Table II. Comparison of the prospectively measured serum liver enzyme values and body measurements.
Table III. Comparison of the prospectively measured serum gonadal hormones.
Table IV. Comparison of the prospectively measured serum lipid values and glucose metabolism.
Table V. Comparison of the prospectively measured magnetic resonance parameters.
Pearson's correlation analysis was performed to assess the correlation between serum blood concentrations and MRI measurements, and correlations were expressed as coefficient r. A p-value of < 0.05 was considered significant. Correlation calculations were performed using IBM SPSS statistics version 20 software package.
Results
Patients
Baseline characteristics of patients are shown in . Median age of the 19 patient was 35 years (range 20–55) and median time interval between the study and initial diagnosis and prior orchiectomy was 3.1 months (0.4–74.6). Mean glomerular filtration rate was 136 ± 39 ml/min. With regard to life style, three patients (15.8%) were smokers and two patients (10.5%) had an alcohol intake of > 20 units/week. Ten patients were classified as having good-risk and nine as intermediate risk metastatic disease according to the international germ cell consensus classification (IGCCC). Histopathology of the tumor showed seminoma (26.3%) in five patients and non-seminoma (73.7%) in 14 patients. Fourteen patients received three and five patients received four courses of BEP chemotherapy. The median (range) cumulative dose administered were 270 (120–360) mg for bleomycin, 650 (515–1000) mg for cisplatin, and 3300 (2550–4200) mg for etoposide. All patients had a favorable antitumor response to treatment; one patient developed pulmonary and arterial emboli to the cerebrum and spleen after his second chemotherapy course and was diagnosed with a hitherto unknown patent foramen ovale and factor-V Leiden mutation.
Anthropometrics
At baseline, seven patients (36.8%) were overweight and one (5.3%) had obesity. Resting blood pressure and heart rate did not change during the follow-up period. However, on average the patients gained weight and BMI increased from 24.3 ± 3.7 kg/m2 at baseline, to 24.7 ± 4.6 kg/m2 (N.S.) and 26.4 ± 4.1 kg/m2 (p = 0.001), respectively, at three and nine months.
Laboratory investigations
The routine liver chemistry parameters including transaminase, γ-GT remained unchanged at the follow-up moments. In contrast, AP and LDH (also a marker for TC disease activity) were decreased at the follow-up measurements ().
Median serum concentrations of the gonadotropins LH and FSH markedly increased after the start of chemotherapy with serum LH significantly rising from 4.9 U/l (0.1–20.9) to 13.2 U/l (5.9–30.4; p = 0.004) at three months after chemotherapy and this increase was sustained at nine months after chemotherapy [12.5 (8.0–28.5) U/l; p = 0.003]. Similarly, median FSH serum concentrations rose from 7.5 U/l (0.1–89.0) at baseline, to 22.3 U/l (9.5–45.0; p = 0.03) at three months and 20.3 U/l (8.6–65.8; p = 0.02) at nine months. Median serum SHBG was increased at three months [31.0 (7.7–71.4) vs. 58.1 nmol/l (28.5–149.0); p < 0.001], but returned to baseline at nine months. Testosterone and estradiol, however, remained unchanged ().
Immediately after chemotherapy mean serum total cholesterol (4.88 ± 1.31 vs. 5.61 ± 1.50 mmol/l; p = 0.002) and LDL-C (3.31 ± 1.16 vs. 3.73 ± 1.4 mmol/l; p = 0.02) significantly increased compared to baseline (, ). No significant changes were observed in serum triglyceride and HDL-C concentrations (, ). LDL-C remained increased at nine months (3.31 ± 1.16 vs. 3.82 ± 1.60 mmol/l; p = 0.02), whereas all other lipid parameters returned to baseline values at this late follow-up time point ().
Figure 1. Serum insulin (upper left panel), total cholesterol (upper right panel), LDL-C (lower left panel) and triglyceride (lower right panel) concentrations before, three and nine months after start of chemotherapy. The graphs show a 25th to 75th percentile box, whiskers (5th to 95th percentile) with the median, and a mean with the standard error.
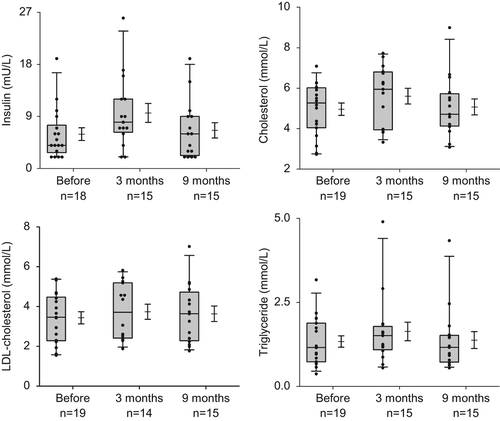
Fasting glucose did not significantly change at the time points measured but insulin significantly increased at three months (5.7 ± 4.4 vs. 9.6 ± 6.3 mU/ml; p = 0.03) and thus HOMA increased (1.33 ± 1.14 vs. 2.11 ± 1.77; p = 0.02). After nine months, insulin and HOMA returned to baseline values (). Least square means estimations showed that while serum glucose levels remained constant, insulin and HOMA increased over time following institution of chemotherapy (). These disturbances in glucose metabolism did not translate into changes in HbA1c, although C-peptide slightly decreased (p = 0.04, ).
Magnetic resonance measurements
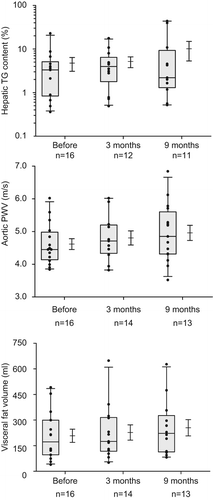
Before chemotherapy administration all aortic pulse wave velocities were in the normal range (4.6 ± 0.7 m/s) and aortic PWV did not change over time with a mean velocity of 5.0 ± 0.8 m/s at three months and 4.7 ± 0.7 m/s at nine months ().
HTC as measured by 1H-MRS before chemotherapy varied (median 2.99; range 0.4–22.8%) with the majority of the values in the normal range. HTC increased, although not significantly, at three months, immediately after cessation of chemotherapy treatment, to a median of 3.51% (0.5–17.6) (; ). At nine months median HTC had decreased to 2.20% (0.5–43.8).
Figure 2. Magnetic resonance measurements of subsequently hepatic triglyceride content (upper panel, semi-logarithmic), aortic PWV (middle panel) and visceral fat volume (lower panel) before, three and nine months after start of chemotherapy. The graphs show a 25th to 75th percentile box, whiskers (5th to 95th percentile) with the median, and a mean with the standard error.
In seven of the 19 (36.8%) patients, three of whom were overweight and one obese, 1H-MRS showed an increase in HTC at either three or nine months. The largest increase in HCT was observed in two of these patients at nine months; HTC increased from 22.3% at three months after start of treatment to 43.8% and from 8.7% at baseline to 39.8%, respectively. Overall, patients with overweight or obesity had significantly higher HTCs than patients with a normal or low BMI (p = 0.002).
HTC correlated with serum triglyceride concentration (r = 0.43, p = 0.004) and serum LDL-C concentration (r = 0.30, p = 0.05), but not with alcohol use. There was no correlation between HTC at three and nine months and cumulative dose of chemotherapy drugs (data not shown).
Mean abdominal subcutaneous fat volume was 556 ± 394 ml at baseline and did not change after three months (594 ± 390 ml) but eventually increased to 668 ± 460 ml (p = 0.002) at nine months. Similarly, abdominal visceral fat volume increased from 202 ± 141 to 237 ± 153 ml (p = 0.009) and 246 ± 170 ml (p = 0.05) at three and nine months, respectively. Increase in visceral abdominal fat volume was correlated to serum HDL-C changes (r = −0.62, p = 0.05) and increase in BMI (r = 0.75, p = 0.003).
Discussion
In our cohort of metastatic but otherwise healthy testicular cancer patients, significant increases of plasma insulin, insulin resistance, total cholesterol and LDL-C, BMI, and a concomitant increase in the volume of the abdominal visceral and subcutaneous fat mass were observed within months after chemotherapeutic treatment. These measured changes in serum lipid concentrations in combination with an increase of visceral fat volume seem to reflect a change in fat metabolism during or shortly after chemotherapy administration. Serum gonadotropins increased significantly after chemotherapy while serum testosterone and SHBG remained unchanged. No statistically significant changes in HTC or aortic PWV were observed.
To our knowledge, this is the first report on GCT patients undergoing chemotherapy in which HTC was measured using 1H-MRS. We used a landmark to ensure the same position of the voxel during all measurements hepatic TG content to chemotherapy. Theoretically, the measured increase in HTC could be due to focal as opposed to generalized liver fattening, but it seems more likely that intravenously administered chemotherapy indeed induces generalized steatosis of the liver in susceptible patients. The lack of change in serum liver enzymes and other laboratory values at follow-up time points do not suggest the presence of a systemic inflammatory state or drug-induced hepatitis.
Intra-individual variation in response of HTC to chemotherapy could result from differences between patients with respect to daily caloric intake, e.g. due to chemotherapy-associated anorexia and nausea. However, co-administration of effective anti-emetics ensured a constant daily caloric intake in all TC patients, thus excluding this as a possible explanation for observed intra-individual differences. In agreement with observations of Mahmood et al. [Citation25], an increase in HTC was particularly evident in obese patients, suggesting an inherent difference between individuals to respond to toxic agents including alcohol by induction of fattening of the liver [Citation26]. The small number of patients precludes a firm conclusion about whether this finding could result from an a priori increased sensitivity of obese patients to develop metabolic abnormalities after chemotherapy.
Interestingly, Qi et al. recently reported their results of serial 1H-MRS measurements of hepatic lipids in patients with colorectal cancer over the time course of a 24-week chemotherapeutic regimen to determine whether 1H-MRS could be used to monitor the progression of chemotherapy- induced steatosis [Citation27]. Different cytotoxic agents and routes of administration were part of the chemotherapy regimen employed; some patients received intravenously administered agents whereas others underwent hepatic arterial infusion with chemotherapy. They observed an increase in the ratio of fat to fat+ water (FFW) after completion of treatment in approximately half of their patients, with both increases and decreases in FFW observed in comparison to baseline. Interestingly, also in this study, patients whose six-week hepatic lipid levels had increased significantly relative to baseline had a high probability of lipid elevation relative to baseline at the completion of treatment.
In contrast with earlier documentation, mean serum testosterone after chemotherapeutic treatment remained unchanged in our small population of GCT patient. However, serum estradiol and gonadotropins increased significantly, reflecting at least partial hypogonadism. The increase in serum estradiol could be the result of an increased tissue aromatization of testosterone in body fat. Chemotherapy-induced (partial) hypogonadism, which may persist for more than two years in approximately two thirds of TC patients, has been supposed to contribute to unfavorable changes in fat and glucose metabolism, obesity and ultimately to the development of MetS. We assessed an acute chemotherapy-induced increase in visceral and subcutaneous fat volume, dyslipidemia evidenced by increases in serum total cholesterol and LDL-C, and insulin resistance. These early increases in body weight, visceral and subcutaneous fat volume, together with the dyslipidemia and insulin resistance observed shortly after chemotherapy may potentially also contribute to the reported increased prevalence of MetS and CVD in long-term TC survivors. Increased abdominal visceral fat has indeed been repeatedly shown to correlate with a complex of pro-diabetic and pro-CVD characteristics supporting this hypothesis [Citation28]. An additional factor that may affect the fat metabolism is the use of corticosteroids as part of the antiemetic regimen administered concomitantly with chemotherapy.
We could not demonstrate any significant changes in vascular stiffness as measured by aortic PWV. This is surprising because chemotherapy like cisplatin, bleomycin and paclitaxel have all been reported to acutely cause endothelial damage in vitro, in animal models and in TC patients [Citation13,Citation29] suggesting that chemotherapy-induced endothelial damage could potentially result in an immediate increase in vascular stiffness, aortic PWV blood pressure and heart rate. Also, high blood glucose levels and insulin resistance have been found to be associated with vascular stiffness suggesting that hyperglycemia plays an important role in the pathogenesis of atherosclerosis and cardiovascular complications [Citation30]. Short-term changes in aortic PWV could also result from a lack of balance between sympathetic and parasympathetic nerve activity [Citation31]. The lack of significant change in aortic PWV suggests that either more time may be required to develop vascular stiffness or that long-term vascular abnormalities reported in testicular cancer survivors [Citation6,Citation32] are not accompanied by aortic wall stiffness. We cannot exclude a vascular effect due to the relatively small number of patients or because we used the wrong test to measure vascular changes. Another possibility is that more endothelial damage was induced by administration of chemotherapy than could be measured as acute vascular resistance with aortic PWV measurements.
This prospective single center study has strengths and weaknesses, one of the limitations being the number of patients in this exploratory study testing feasibility of 1H-MRS measurements in patients undergoing chemotherapy. We did not perform 1H-MRS at similar time points in healthy controls, who did not undergo chemotherapy, but instead used patients as their own controls. Randomization of patients between chemotherapy and placebo infusions would not be ethical. The design of the study thus precluded to differentiate between effects of treatment and time. Although chemotherapy is most likely the culprit of observed insulin resistance, dyslipidemia and immediate increase in abdominal visceral and subcutaneous adipose tissue in individual patients, we cannot differentiate between effects mediated by chemotherapy or changes in lifestyle, food intake or corticosteroids (as part of the anti-emetic regimen).
It is known that high-dose steroids may cause acute, transient periods of hyperglycemia [Citation33]. Consequently, we consider it likely that steroid-induced changes in glucose homeostasis were present during the first half of each chemotherapy course, but we have no evidence that these effects persisted far beyond this episode, when we performed our assessments. Thus, although we cannot fully exclude the influence of steroids on glucose homeostasis, the time interval between last steroid dose and time point of blood measurements suggests that chemotherapy with its more profound inflict on the body's defence mechanisms to be the more likely culprit of the observed insulin resistance. The disadvantage of not being able to unequivocally exclude the contribution of corticosteroids, or changes in lifestyle and food intake is off-set by the benefits of performing our research in routine clinical practice. Furthermore, we used aortic PWV as a surrogate for vascular stiffness instead of measuring endothelial damage itself, this could possibly result in an underestimation of the toxic effect of chemotherapy on the endothelial wall.
The exploratory nature of the study and the complex – if not impossible – logistics of more frequent MRI measurements in patients who required fixed accepted standard schedules of chemotherapy during several subsequent days, precluded assessment at more frequent time points. From an oncological point of view, deviation from accepted curative chemotherapy schedules precluded measurements during the daily long hours of fixed chemotherapy infusions.
In conclusion, standard treatment of testicular cancer patients with BEP chemotherapy immediately results in insulin resistance and dyslipidemia and – most strikingly in obese patients – an increase in liver triglyceride content. The concomitant increase in abdominal subcutaneous and visceral fat volume suggests that indeed chemotherapy acutely affects insulin sensitivity and fat distribution. This implies that, already early after treatment, GCT patients should be screened and subsequently treated for cardiovascular risk factors. Although long-term TC survivors are at risk for hypertension and increased cardiovascular disease, vascular changes cannot be captured by aortic PWV measurements within one year after cisplatin-based chemotherapy. Prospective studies in larger groups of testicular cancer patients will enable to demonstrate if early changes in serum profiles reflecting fat and glucose metabolism and abdominal and hepatic fat content occur more frequently in subsets of patients, relate to individual sensitivity to respond to insults inflicted by chemotherapy and whether they predict for metabolic abnormalities in long-term survivors of testicular cancer.
Acknowledgements
We gratefully acknowledge the contributing efforts of Mrs. Adriana Q. M. J. van Steijn-van Tol, research nurse at the Department of Clinical Oncology and Dr. Sebastiaan Hammer, resident at the Department of Radiology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Einhorn EH. Testicular cancer: An oncological success story. Clin Cancer Res 1997;3:2630–2.
- Nuver J, Smit AJ, Wolffenbuttel BH, Sluiter WJ, Hoekstra HJ, Sleijfer DT, et al. The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol 2005;23:3718–25.
- Haugnes HS, Aass N, Fossa SD, Dahl O, Klepp O, Wist EA, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol 2007;18:241–8.
- Stefenelli T, Kuzmits R, Ulrich W, Glogar D. Acute vascular toxicity after combination chemotherapy with cisplatin, vinblastine, and bleomycin for testicular cancer. Eur Heart J 1988;9:552–6.
- Weijl NI, Rutten MF, Zwinderman AH, Keizer HJ, Nooy MA, Rosendaal FR, et al. Thromboembolic events during chemotherapy for germ cell cancer: A cohort study and review of the literature. J Clin Oncol 2000;18:2169–78.
- Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol 2003;21:1513–23.
- Pollera CF, Ameglio F, Nardi M, Vitelli G, Marolla P. Cisplatin-induced hepatic toxicity. J Clin Oncol 1987;5: 318–9.
- Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991;14:1132–43.
- Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol 2001;88:1264–9.
- Despres JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol 2007;6:51–9.
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis 2007;17:319–26.
- Montiel M, Urso L, de la Blanca EP, Marsigliante S, Jimenez E. Cisplatin reduces endothelial cell migration via regulation of type 2-matrix metalloproteinase activity. Cell Physiol Biochem 2009;23:441–8.
- Nuver J, de Haas EC, van Zweeden M, Gietema JA, Meijer C. Vascular damage in testicular cancer patients: A study on endothelial activation by bleomycin and cisplatin in vitro. Oncol Rep 2010;23:247–53.
- Heier MS, Nilsen T, Graver V, Aass N, Fossa SD. Raynaud’s phenomenon after combination chemotherapy of testicular cancer, measured by laser Doppler flowmetry. A pilot study. Br J Cancer 1991;63:550–2.
- Fischbach F, Bruhn H. Assessment of in vivo 1H magnetic resonance spectroscopy in the liver: A review. Liver Int 2008;28:297–307.
- van der Meer RW, Hammer S, Lamb HJ, Frolich M, Diamant M, Rijzewijk LJ, et al. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab 2008;93:2702–8.
- Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722–8.
- De Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA. The metabolic syndrome in cancer survivors. Lancet Oncol 2010;11:193–203.
- Amar J, Ruidavets JB, Chamontin B, Drouet L, Ferrieres J. Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens 2001;19:381–7.
- Maldonado J, Pereira T, Polonia J, Silva JA, Morais J, Marques M. Arterial stiffness predicts cardiovascular outcome in a low-to-moderate cardiovascular risk population: The EDIVA (Estudo de DIstensibilidade VAscular) project. J Hypertens 2011;29:669–75.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53.
- van der Meer RW, Doornbos J, Kozerke S, Schar M, Bax JJ, Hammer S, et al. Metabolic imaging of myocardial triglyceride content: Reproducibility of 1H MR spectroscopy with respiratory navigator gating in volunteers. Radiology 2007; 245:251–7.
- Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462–8.
- Mahmood S, Taketa K, Imai K, Kajihara Y, Imai S, Yokobayashi T, et al. Association of fatty liver with increased ratio of visceral to subcutaneous adipose tissue in obese men. Acta Med Okayama 1998;52:225–31.
- Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, et al. Hepatic lipid accumulation in healthy subjects: A comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med 2006;55:913–7.
- Qi J, Fong Y, Saltz L, D’Angelica MI, Kemeny NE, Gonen M, et al. Serial measurement of hepatic lipids during chemotherapy in patients with colorectal cancer: A 1 H MRS study. NMR Biomed 2013;26:204–12.
- Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, et al. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med 1989; 110:867–72.
- Kohn S, Fradis M, Podoshin L, Ben-David J, Zidan J, Robinson E. Endothelial injury of capillaries in the stria vascularis of guinea pigs treated with cisplatin and gentamicin. Ultrastruct Pathol 1997;21:289–99.
- Webb DR, Khunti K, Silverman R, Gray LJ, Srinivasan B, Lacy PS, et al. Impact of metabolic indices on central artery stiffness: Independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010; 53:1190–8.
- van der Meer RW, Hammer S, Smit JW, Frolich M, Bax JJ, Diamant M, et al. Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes 2007;56:2849–53.
- Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: A 20-year follow-up study. J Clin Oncol 2010;28:4649–57.
- Fong AC, Cheung NW. The high incidence of steroid- induced hyperglycaemia in hospital. Diabetes Res Clin Pract 2013;99:277–80.
