Abstract
Background. Worldwide marked changes have been observed in the incidence and survival of testicular cancer (TC) during the last decades. We conducted a study on trends in TC incidence, treatment, survival, and mortality in the Netherlands during the period 1970–2009 with specific focus on trends according to age, histology and stage of disease. Methods. Data from the Eindhoven cancer registry, the Netherlands cancer registry and Statistics Netherlands was used. Age-standardized incidence and mortality rates and five-year relative survival were calculated. Treatment was categorized into five major groups. Results. TC incidence showed a substantial annual increase of 3.9% in the period 1989–2009. The incidence increased for all stages of both seminoma and non-seminoma TC. Stage distribution for the non-seminoma patients shifted towards more localized disease. Most patients received primary treatment according to the guidelines. Five-year relative survival improved (non-significantly) for most groups of stage and histology. TC mortality dropped sharply in the 1970s and 1980s and remained relatively stable thereafter. Conclusion. This study shows that incidence of TC has increased sharply in the Netherlands. Relative survival is high and improved in most disease stages. There is a growing demand for medical care of newly diagnosed TC patients and for the rapidly increasing number of prevalent TC patients.
Testicular cancer (TC) is the most commonly diagnosed cancer among men aged 20–39 years in the Netherlands [Citation1]. Ninety-five percent of all TCs are germ cell tumors, which can further be divided into seminomas and non-seminomas [Citation2].
The etiology of TC is only partly understood. Cryptorchidism, a contralateral TC and a family history of TC are the best established risk factors [Citation2]. These factors can not, however, explain the increase in TC incidence that has been observed in most developed countries during the past 50 years [Citation3–7].
Besides an increased incidence, survival of TC has also improved. A study in the Southern part of the Netherlands showed that 10-year relative survival of seminoma and non-seminoma patients increased from 81% and 54%, respectively, in the 1970s to over 90% in the 1990s for both histologies [Citation8]. This improvement in survival is mainly due to the introduction of cisplatin-based chemotherapy in the late 1970s [Citation8,Citation9]. This also resulted in a steep decrease in mortality in most European countries since the 1970s [Citation7].
To evaluate recent progress against TC in the Netherlands we conducted a study on trends in TC incidence, treatment, survival and mortality during the period 1970–2009 with specific interest in the trends according to age, histology and stage.
Methods
Population-based data from 1989 onwards from the nationwide Netherlands Cancer Registry (NCR) were used, specific details of the registration methods of the NCR have been described elsewhere [Citation10]. As there was no nationwide cancer registry in the Netherlands before 1989, we have used data of the Eindhoven Cancer Registry (ECR) to investigate trends in incidence between 1970 and 1989. The ECR is a registry in the south of the Netherlands, which was already started in the 1950s and is considered to be complete since 1970 [Citation8,Citation11,Citation12]. The ECR covered about 7% of the Dutch population in the period 1970–1989. National mortality data for the period 1970–2009 was obtained from Statistics Netherlands.
All patients with invasive primary TC (i.e. in situ tumors were not included) diagnosed during the period 1989–2009 in the Netherlands were included in the analyses. We excluded all hematological tumors of the testis (e.g. lymphomas) from our analyses (n = 521), as these are generally not classified as testicular cancers. The tumors were grouped according to histological origin: seminomas (ICD-O-3 codes:9060-9064), non-seminomas (9065-9085, 9100-9102,9105) or other (including: Leydig and Sertoli cell tumors, sarcomas, and not otherwise specified tumors) [Citation13]. Pathological TNM (pTNM) stage was used, for cases in which pN (90%) and/or pM (38%) were unknown clinical stage (cN and/or cM) were used. Stage was classified as localized (T1-4, N0/Nx, M0/Mx or TX, N0, M0), regional lymph nodes (any T, N+, M0/Mx), distant metastases (any T, any N, M1) and stage unknown (Tx, N0, Mx or Tx, Nx, M0 or Tx, Nx, Mx). Patients with stage unknown (n = 196, 1.9%) were excluded from analyses according to stage.
Patients aged ≤ 15 years (n = 77) and cases diagnosed by autopsy (n = 2) were excluded from survival analyses. The younger patients were excluded because there were not enough patients in each period to calculate relative survival according to period.
As treatment is directly recorded from the medical files by the registration clerks it is generally regarded to have a good accuracy. There were, however, some doubts about three patient groups (patients who received no surgery (including no orchidectomy), patients with distant metastases who only underwent surgery (i.e. no chemotherapy or radiotherapy) and patients with a non-seminoma tumor who received radiotherapy. The registered treatments of all of these patients underwent an additional manual check by registration clerks in the medical files and were changed if necessary.
Statistical analyses
Three-year moving average age-standardized incidence and mortality rates [European Standardized Rates (ESR)] were calculated per 100 000 person-years. For the overall three-year moving average age-standardized incidence rates of TC and for the three-year moving average age-standardized incidence rates of the seminomas and non-seminomas data of the ECR was used for the period 1970–1989 and data of the NCR for the period 1990–2009. For all age- and stage-specific incidence analyses, only data of the NCR was used. Changes in incidence were evaluated by calculating the estimated annual percentage change (EAPC) with corresponding 95% confidence interval (CI).
Primary treatment of TC was divided into the following groups: surgery alone, surgery and radiotherapy, surgery and chemotherapy, surgery, radiotherapy and chemotherapy and no surgery (with or without radiotherapy or chemotherapy). Surgery includes all types of surgery (e.g. orchidectomy, retroperitoneal lymph node dissection, resection of residual masses). Received treatment is presented as percentages per period according to histology and stage. The Cochran-Armitage trend test was used to test for differences over time.
Relative survival was calculated as the time from diagnosis to death, emigration or December 31, 2009. Relative survival is an estimation of the disease-specific survival. It is calculated as the absolute survival amongst cancer patients divided by the expected survival for the general population with the same sex and age structure. Traditional cohort-based relative survival analysis was used to calculate five-year relative survival. For the five-year survival estimates of the last period (2004–2009) only the patients diagnosed in 2004 had five-year follow-up. Recent changes in survival might therefore not be accurately represented by standard cohort five-year survival estimates. Period-based relative survival analysis should provide the most up-to-date estimates for recent time periods [Citation14]. A sensitivity analysis was performed to check whether the cohort-based survival estimates were similar to the period-based relative survival estimates for the most recent period. The five-year cohort-based relative survival estimates for the four time periods were used in a Poisson model to test the significance of increases or decreases over time. All tests were performed two-sided, p < 0.05 was considered to be significant.
Results
Incidence and mortality
Between 1989 and 2009, 10 384 cases of TC were diagnosed in the Netherlands (). The annual number of cases doubled from 336 in 1989 to 667 in 2009. The age-standardized incidence rate in the region of the ECR remained relatively stable during the 1970s and 1980s (EAPC = 0.2%, 95% CI –2.4–2.7%) (). From 1989 onwards there was a sharp increase in TC incidence with an EAPC of 3.9% (95% CI 3.6–4.3%) from 1989 to 2009. From 1992 onwards the incidence rates of the ECR and NCR were similar (data not shown). The incidence of both seminomas and non-seminomas showed similar increases from 1989 onwards with EAPCs of 3.7% (95% CI 3.2–4. 2%) and 4.3% (95% CI 3.8–4.8%), respectively.
Figure 1. Three-year moving average European standardized (ESR) incidence and mortality rates for testicular cancer in the Netherlands 1970–2009 per 100 000 person-years.
(Incidence rates 1970–1989: data from the Eindhoven Cancer Registry; Incidence rates 1990–2009: data from the Netherlands Cancer Registry; mortality rates 1970–2009: Statistics Netherlands).
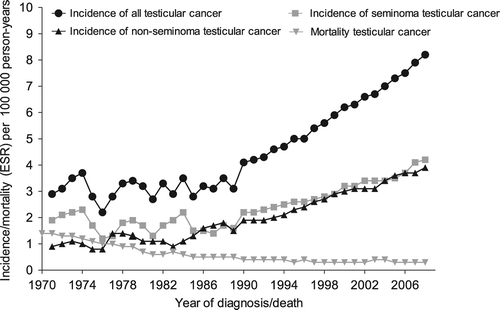
Table I. Patient and tumor characteristics of testicular cancer patients included in this study.
The age-standardized mortality dropped from 1.4 per 100 000 person-years in 1970 to around 0.3 in the mid 1990s and remained relatively stable thereafter.
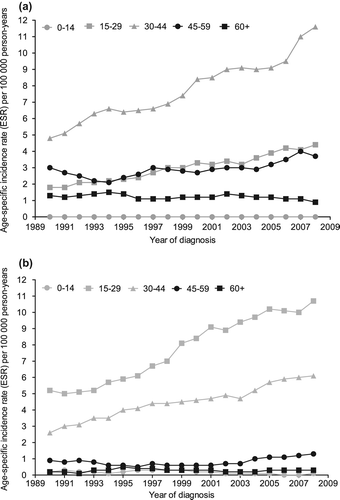
Age-specific incidence
For both histologies the age-groups of 15–29 and 30–44 years showed the largest increases in incidence (), with EAPCs between 4.4% and 5.1%. The incidence rates among men aged 45–59 years also exhibited a significant increase for both seminoma (EAPC = 1.9%, 95% CI 0.6–3.2%) and non-seminoma (EAPC = 2.7%, 95% CI 0.1–5.22%) patients. The incidence of non-seminoma patients of ≤ 14 years showed a significant decrease (EAPC = −6.3%, 95% CI −10.7–−1.8%), but this was based on only 50 patients.
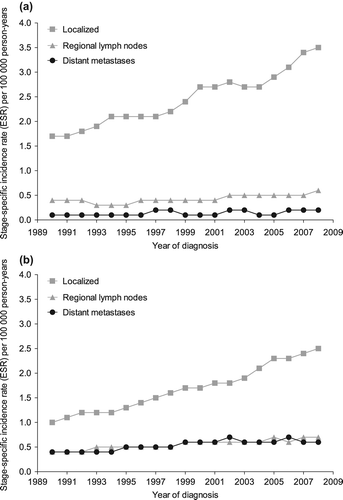
Stage-specific incidence of seminomas
In the period 1989–1993, 78% of the seminoma patients were diagnosed with localized disease, 15% with regional lymph node involvement, 5% with distant metastases, and 1.8% with an unknown stage (data not shown). In the period 2004–2009, these percentages were 81%, 14%, 5%, and 0.2%, respectively. The incidence of localized seminomas increased from 1.7 per 100 000 person-years in 1989 to 3.4 in 2009 (EAPC = 3.9%, 95% CI 3.4–4.5%) (). There was a somewhat smaller increase in seminomas with positive regional lymph nodes (EAPC = 2.9%, 95% CI 1.9–4.0%). The incidence rate for seminoma patients with distant metastases increased from 0.1 per 100 000 person-years in 1989 to 0.2 in 2009 (EAPC = 4.5, 95% CI 0.8–8.3%).
Stage-specific incidence of non-seminomas
The percentage of patients with localized (clinical stage I) non-seminoma increased from 57% in the period 1989–1993 to 64% in the period 2004–2009, the percentage of regional lymph nodes decreased from 22% to 19%; distant metastases decreased from 21% to 17% and the percentage with an unknown stage decreased from 0.8% to 0.3% (data not shown). The incidence of localized non-seminomas increased from 1.0 per 100 000 person-years in 1989 to 2.6 in 2009, with an EAPC of 5.2% (95% CI 4.6–5.8%) (). The incidence rates for patients with positive regional lymph nodes and patients with distant metastases also increased significantly, with EAPCs of 3.3% (95% CI 2.2–4.4%) and 2.7% (95% CI 1.5–3.8%), respectively.
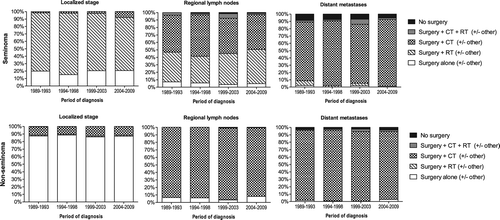
Treatment of seminoma
Treatment of localized seminoma TC varied over time (). The percentage of patients who only underwent surgery varied between 15% and 21%, while the percentage of patients who received surgery and radiotherapy decreased from 78% in the period 1989–1993 to 71% in the period 2004–2009 (p < 0.0001). The percentage of patients who received surgery and chemotherapy increased from 1.5% to 7.9% (p < 0.001). Almost all seminoma TC patients with regional lymph node involvement underwent either surgery and radiotherapy (varied between 36% and 46%) or surgery and chemotherapy (47–55%), with no significant changes over time. The percentage of seminoma TC patients with distant metastases who received surgery and chemotherapy increased from 80% to 91% (p = 0.15) and fewer of these patients underwent no surgery (decreased from 9% to 5%).
Treatment of non-seminoma
Of the patients with localized non-seminoma 86–89% received only surgery. Most of the remaining patients underwent surgery and chemotherapy, without any significant changes over time. More than 91% of the non-seminoma TC patients with regional lymph nodes and 92% of the non-seminoma patients with distant metastasis received surgery and chemotherapy.
Survival
The overall five-year relative survival of TC improved from 95% in 1989–1993 to 98% in 2004–2009 (p < 0.0001) (). The survival of patients aged 15–29 and 30–44 years improved significantly from 95% to 98% and from 96% to 98%, respectively. Survival of the oldest two age groups also increased over time, but not significantly. Patients aged ≥ 60 years even exhibited the largest improvement in survival, from 85% to 96% (p = 0.86).
Table II. Five-year relative survival with 95% confidence intervals for patients with testicular cancer in the Netherlands according to period of diagnosis, age and histology and stage groups.
Survival of seminoma
Patients with a localized seminoma had a high five-year relative survival throughout the whole study period (99–101%) (). Survival of seminoma patients with regional lymph node metastases improved significantly from 93% to 100%. Five-year relative survival of patients with distant metastases improved from 73% to 88% (p = 0.07).
Survival of non-seminoma
The five-year relative survival for patients with a localized tumor was 98–99% (). Survival of non-seminoma patients with regional lymph node metastases varied between 94% and 98%, with no significant trend. Five-year relative survival of patients with distant metastases improved from 78% to 85% (p = 0.05).
Comparison of cohort- and period-based survival analysis
Cohort- and period-based five-year relative survival estimates were compared for the period 2004–2009. Only the survival estimates of patients aged ≥ 60 years showed differences larger than two percentage points between the cohort- and period-based five-year relative survival estimates. The estimate of the cohort-based analysis of patients aged ≥ 60 years was 95.7% [Standard Error (SE) = 5.5%], while the period-based survival estimate was 91.6% (SE = 4.3%). We chose to present cohort-based survival estimates in all other analyses for the period 2004–2009, so that all relative survival estimates are cohort-based and thus comparable.
Discussion
There was a marked and continuing increase in TC incidence in the Netherlands during the period 1989–2009, the largest increases were seen for patients with localized disease. There was little variation over time in the treatment patterns for the different histologies and stages of TC. Relative survival of most stages improved slowly over time, however, only the improvement of survival of patients with seminoma with regional lymph node metastases was significant.
The incidence rate of TC as well as the increase in incidence in the Netherlands is similar to that in other industrialized countries [Citation3,Citation4,Citation6,Citation7,Citation12]. While, in other industrialized countries, the incidence started to increase during the 1960s–1970s, it was not until the late 1980s that it started to increase in the Netherlands [Citation3,Citation4,Citation6,Citation7,Citation12]. This may have been caused by calendar differences in the onset of exposure to yet unknown risk factors. This trend difference may thereby help to identify risk factors.
A possible explanation for this time difference might be that in the Netherlands, in contrast to other Western countries, the emancipation of women (increase in age at first birth, the decrease in the number of children per woman and the increasing use of alcohol and tobacco by women, etc.) started relatively late and some factors associated with the emancipation, such as sibship size and maternal age, are possibly related to TC [Citation15].
An important established risk factor for TC is cryptorchidism, although it is unclear whether this predisposes to TC or whether it shares common risk factors with TC [Citation15]. The testicular dysgenesis syndrome (TDS) hypothesis suggests that four conditions (cryptorchidism, hypospadias, impaired spermatogenesis and TC) are associated with each other as different manifestations of disturbed prenatal testicular development [Citation16]. In utero or perinatal exposure to endocrine disrupters (exogenous estrogens and anti-androgens) is the presumed exogenous exposure for the development of TDS [Citation16,Citation17]. However, if the TDS hypothesis is true, the different conditions of this syndrome should exhibit similar trends in incidence. While it is clear that the incidence of TC has been increasing in most developed countries, it is not clear whether the incidence of cryptorchidism and hypospadias has increased similarly [Citation18]. Due to the complexity of the pathogenic and epidemiologic features of each component of the TDS it will probably take a while before this hypothesis is finally proven or disproven.
Other important established determinants of TC are familial occurrence and a contralateral testicular tumor [Citation2,Citation15,Citation19,Citation20]. As the genetic make-up of a stable population cannot change very rapidly and most TCs are still detected in men without a history of TC, these risk factors cannot have caused the large and rapid increase of TC incidence. Other factors such as low birth weight, low gestational age and low and high maternal age might also influence the risk of TC [Citation15]. Although a considerable amount of etiological research has been performed, the underlying cause for the increase of TC incidence remains poorly understood [Citation2,Citation19,Citation20].
Several other studies also observed an increase in incidence of TC that was more marked for localized than for disseminated stages or an increasing percentage of localized stages [Citation21–24]. A shift towards more localized disease could be due to several reasons.
Improved education and awareness of TC and cancer in general among patients and general practitioners could result in earlier detection of the tumor and thus a shift towards a higher percentage of localized tumors. A recent Irish study showed that awareness and knowledge of TC has indeed increased among men [Citation25].
The increase in localized tumors could also be due to changes in case-finding practices of general practitioners and urologists or the use of more sensitive imaging modalities. Although there seems to be an increase in the utilization of echo imaging for scrotal complaints, it is however unlikely that this alone could cause the large increase in TC incidence.
The introduction of a new risk factor for TC could also have played a role, especially if this risk factor would cause more slower growing tumors. Because of the limited knowledge of the etiology of TC, it is impossible to test this hypothesis.
It is not unlikely that a combination of the above-mentioned explanations is responsible for the increased incidence of localized tumors.
The primary treatment for TC has been rather clear for some time and is well described in Dutch and European guidelines [Citation26–28]. Orchidectomy is the start of treatment for all stages of TC. For stage I seminoma TC there are three treatment options after orchidectomy, i.e. surveillance, adjuvant radiotherapy of retroperitoneal para-aortic lymph nodes or one cycle of adjuvant carboplatin [Citation26]. For stage I non-seminoma TC the standard option after orchidectomy in the Netherlands is surveillance, although until recently some hospitals performed a RPLND after orchidectomy routinely [Citation26]. Seminoma TC patients with stage IIA/IIB (regional lymph nodes up to 5 cm) usually undergo radiotherapy to the para-aortic and ipsilateral iliac lymph nodes [Citation27]. All other patients with disseminated seminoma or non-seminoma TC should receive BEP (bleomycin, etoposide and cisplatin) chemotherapy [Citation27].
Although the large majority (71–83%) of the patients with a localized seminoma underwent surgery and adjuvant radiotherapy, there was a significant trend towards more surgery and chemotherapy. More than 86% of the patients with a localized non-seminoma underwent only surgery.
Since we could not stratify the group of patients with regional lymph nodes according to the size of the lymph nodes, we found two large treatment groups for seminoma patients with nodal involvement. About 36–46% of these patients underwent surgery and radiotherapy and 47–57% of the patients underwent surgery and chemotherapy. More than 91% of the non-seminoma patients with positive regional lymph nodes received chemotherapy, while 3–8% of the patients underwent only surgery, probably consisting of orchidectomy and RPLND.
Of the seminoma patients with distant metastases 80–91% received surgery and chemotherapy. For the non-seminoma patients this varied between 92% and 95%.
There were 43 (3.6%) patients with distant metastases who did not undergo any form of surgery, 17 of these patients were diagnosed with a seminoma and 26 with a non-seminoma. Of these patients, 42 (98%) had a histological confirmation of the disease, 32 (74%) only received chemotherapy treatment, while seven (16%) received no treatment. The patients had a relatively high age (mean age was 44 years) and a poor survival (i.e. 20 patients died within three months after diagnosis).
Although this study could not present detailed data on treatment, most patients seem, in general, to have been treated according to the guidelines.
Except for the non-seminoma patients with distant metastases, the relative survival was quite similar to that of American patients with comparable histology and stage who were diagnosed between 1988 and 2001 [Citation29]. For the Dutch non-seminoma patients with distant metastases the five-year relative survival improved from 78% to 85% over the study period (p = 0.05), while it was 72% for the American patients [Citation29]. The improvement of the survival in the Netherlands is likely due to improved chemotherapy and the referral of patients with metastasized TC to specialized centers. The increase in survival of non-seminoma patients with distant metastases can however also be due to other reasons, such as improvements of the postchemotherapy RPLND or a stage shift within the group non-seminoma patients with distant metastases (from the poor and intermediate IGCCC prognosis groups towards the intermediate and good IGCCC prognosis groups). The somewhat lower survival in USA for the distant metastases in contrast to the Netherlands might be explained by the disparities in stage distribution and relative survival that exist between different racial/ethnic groups in the USA. This might be affected by differences in socioeconomic status, cultural and lifestyle factors, health insurance coverage, and healthcare access and usage [Citation30]. Most of these factors are more homogeneous in the Netherlands.
A limitation of our study is that the cohort- survival estimate for the relative five-year survival of the period 2004–2009 is largely dependent on the patients diagnosed in 2004. However, the cohort- and period-based five-year relative survival estimates exhibited relatively small differences. We therefore can expect that the true five-year relative survival in this time period resemble the estimates reported in this study. Another limitation of this study is that there is no nationwide data on the incidence of TC prior to 1989. As the incidence rates of the ECR and NCR are very similar for the period 1992–2009, we assume that the trend in incidence rate in the whole of the Netherlands prior to 1989 is the similar as in the region of the ECR.
In conclusion, incidence of TC has increased sharply in the Netherlands over time, with the largest increase in localized tumors, relative survival remains high and mortality is low. There is a growing demand for medical care for newly diagnosed TC patients and the rapidly increasing number of prevalent TC patients who require a long active follow-up and might experience long-term side-effects of the radiotherapy and chemotherapy treatment.
Acknowledgements
This work was performed as part of the project ‘Progress against cancer in the Netherlands since the 1970s?’ (Dutch Cancer Society EMCR 2006-3489). The authors thank the registration clerks for dedicated data collection. Rob Verhoeven was supported by the Comprehensive Cancer Centre South.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- NCR. Netherlands Cancer Registry. [cited 2011 Sep 6]. Available from: http://www.cijfersoverkanker.nl
- Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. Br J Urol Int 2009;104:1329–33.
- Holmes L, Jr., Escalante C, Garrison O, Foldi BX, Ogungbade GO, Essien EJ, et al. Testicular cancer incidence trends in the USA (1975–2004): Plateau or shifting racial paradigm?. Public Health 2008;122:862–72.
- Huyghe E, Plante P, Thonneau PF. Testicular cancer variations in time and space in Europe. Eur Urol 2007;51: 621–8.
- Bray F, Ferlay J, Devesa SS, McGlynn KA, Moller H. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nature Clin Pract 2006;3: 532–43.
- Jacobsen R, Moller H, Thoresen SO, Pukkala E, Kjaer SK, Johansen C. Trends in testicular cancer incidence in the Nordic countries, focusing on the recent decrease in Denmark. Int J Androl 2006;29:199–204.
- Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Moller H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. Int J Cancer 2006;118:3099–111.
- Verhoeven RH, Coebergh JW, Kiemeney LA, Koldewijn EL, Houterman S. Testicular cancer: Trends in mortality are well explained by changes in treatment and survival in the southern Netherlands since1970. Eur J Cancer 2007;43: 2553–8.
- Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A 2002;99:4592–5.
- van Steenbergen LN, Elferink MA, Krijnen P, Lemmens VE, Siesling S, Rutten HJ, et al. Improved survival of colon cancer due to improved treatment and detection: A nationwide population-based study in The Netherlands 1989–2006. Ann Oncol 2010;21:2206–12.
- Coebergh JWW, Janssen-Heijnen MLG, Louwman WJ, Voogd AC, eds. Cancer incidence, care and survival in the South of the Netherlands, 1955-1999: A report of the Eindhoven Cancer Registry with cross border implications. Eindhoven: Comprehensive Cancer Centre South (IKZ); 2001.
- Verhoeven R, Houterman S, Kiemeney B, Koldewijn E, Coebergh JW. Testicular cancer: Marked birth cohort effects on incidence and a decline in mortality in southern Netherlands since 1970. Int J Cancer 2008;122:639–42.
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology, 3rd ed. Geneva: World Health Organization; 2000.
- Brenner H, Gefeller O. An alternative approach to monitoring cancer patient survival. Cancer 1996;78:2004–10.
- McGlynn KA, Cook MB. Etiologic factors in testicular germ-cell tumors. Future Oncol 2009;5:1389–402.
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum Reprod 2001; 16:972–8.
- Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: Foetal origin of adult reproductive problems. Clin Endocrinol 2009;71:459–65.
- Thorup J, McLachlan R, Cortes D, Nation TR, Balic A, Southwell BR, et al. What is new in cryptorchidism and hypospadias – a critical review on the testicular dysgenesis hypothesis. J Pediat Surg 2010;45:2074–86.
- Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World J Urol 2004;22:2–14.
- Garner MJ, Turner MC, Ghadirian P, Krewski D. Epidemiology of testicular cancer: An overview. Int J Cancer 2005;116:331–9.
- Sonneveld DJ, Hoekstra HJ, Van Der Graaf WT, Sluiter WJ, Schraffordt Koops H, et al. The changing distribution of stage in nonseminomatous testicular germ cell tumours, from 1977 to 1996. Br J Urol Int 1999;84:68–74.
- Powles TB, Bhardwa J, Shamash J, Mandalia S, Oliver T. The changing presentation of germ cell tumours of the testis between 1983 and 2002. Br J Urol Int 2005;95:1197–200.
- Shah MN, Devesa SS, Zhu K, McGlynn KA. Trends in testicular germ cell tumours by ethnic group in the United States. Int J Androl 2007;30:206–13; discussion 13–4.
- Heimdal K, Fossa SD, Johansen A. Increasing incidence and changing stage distribution of testicular carcinoma in Norway 1970–1987. Br J Cancer 1990;62:277–8.
- Casey RG, Grainger R, Butler MR, McDermott TE, Thornhill JA. Public awareness of testis cancer and the prevalence of testicular self-examination-changing patterns over 20 years. Urology 2010;76:915–8.
- Krege S, Beyer J, Souchon R, Albers P, Albrecht W, Algaba F, et al. European consensus conference on diagnosis and treatment of germ cell cancer: A report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): Part I. Eur Urol 2008;53:478–96.
- Krege S, Beyer J, Souchon R, Albers P, Albrecht W, Algaba F, et al. European consensus conference on diagnosis and treatment of germ cell cancer: A report of the second meeting of the European Germ Cell Cancer Consensus Group (EGCCCG): Part II. Eur Urol 2008;53:497–513.
- Oncoline. [cited 2011 Sep 26]. Available from: http://www.oncoline.nl/testiscarcinoom
- Biggs ML, Schwartz SM. Chapter 21, Cancer of the testis. In: Ries LAM, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ, editors. SEER survival monograph: Cancer survival among adults: US SEER Program, 1988–2001, Patient and tumor characteristics. Bethesda, MD: National Cancer Institute, SEER Program; 2007.
- Biggs ML, Schwartz SM. Differences in testis cancer survival by race and ethnicity: A population-based study, 1973–1999 (United States). Cancer Causes Control 2004;15:437–44.