Abstract
Background. Concurrent chemo-radiotherapy (CON-CRT) is recommended for selected patients with stage III non-small cell lung cancer (NSCLC), but utilization varies. We assessed the response to national guidelines introduced in 2004 and the impact on outcomes. Material and methods. Retrospective study of stage III NSCLC patients treated with radical intent non-surgical treatment during 2003–2010 in a university medical center characterized by multidisciplinary assessment, routine use of four-dimensional computed tomography for radiotherapy planning, and rapid implementation of radiotherapy advances. Results. Between 2003 and 2010, 319/435 (73%) patients with stage III NSCLC received (chemo) radiotherapy. The number receiving CON-CRT in successive two-year periods increased from 13/48 (27%) – 40/80 (50%) – 63/90 (70%), to 74/101 (73%). Median overall survival (OS) from start of radiotherapy was 18.6 months for CON-CRT (190/319) and 17.4 months for sequential (SEQ), typically hypofractionated, CRT (90/319) (p = 0.78). Eleven months OS with radiotherapy alone (39/319) was significantly shorter (p = 0.006). OS did not differ between the four periods (p = 0.87). CON-CRT was not over-represented in the 16% of patients dying within five months of starting radiotherapy. Conclusions. Between 2003 and 2010, CON-CRT for stage III NSCLC was rapidly and safely increased. However, OS did not increase and, as practiced, did not differ between CON- or SEQ-CRT.
Approximately 80% of patients with lung cancer have non-small cell lung cancer (NSCLC) and about 30% of these present with locally advanced, stage III disease. Concurrent chemo-radiotherapy (CON-CRT) has been associated with better overall survival (OS) than sequential chemo-radiotherapy (SEQ-CRT) or radiotherapy (RT) alone and is generally recommended in selected, fit patients, with a performance status of 0 or 1 [Citation1,Citation2]. However, it is accompanied by higher rates of treatment-related mortality and high-grade esophagitis [Citation3–5]. Dutch national guidelines recommending CON-CRT in selected patients with stage III NSCLC have been available since 2004, and were updated in 2011 [Citation6,Citation7]. In the Netherlands the use of CON- or SEQ-CRT increased from 18% in 2001 to 29% in 2006, but with significant variation between hospitals [Citation8]. A similar study in British Columbia suggests an increase from 8% in 2000 to 18.6% in 2007 (5.1 and 17.6%, respectively, for CON-CRT) [Citation9].
In order to assess the speed, magnitude and impact of changes in patterns of care for stage III NSCLC in response to national guidelines, we retrospectively reviewed the use of high-dose RT with and without chemotherapy in the definitive non-surgical treatment of stage III NSCLC during 2003–2010.
Material and methods
The primary aims of this retrospective study were to describe trends in the management of stage III NSCLC, including the utilization of CON-CRT, and patient survival. Secondary aims included a description of RT-specific characteristics and early mortality. The study was conducted with institutional approval.
Stage III NSCLC patients receiving radical intent, non-surgical treatment at our center between January 2003 and October 2010 were retrospectively identified. All patients received RT at our institute. Chemotherapy was either delivered at the same institute or at a neighboring, regional facility. Patients receiving trimodality therapy or stereotactic RT were excluded. ICD code and codes for CON-CRT, SEQ-CRT and RT were used to select patients from a database recording all patients receiving treatment in the Department of Radiotherapy, (ARIA Oncology Information System, Varian Medical Systems, Palo Alto, USA). All patients had a planned RT dose of at least 39 Gy. The RT and pulmonology charts were obtained and reviewed. Missing data was searched for in a surgical database and an existing radiotherapy department database [Citation10].
All patients were treated in a university medical center by an experienced multidisciplinary team. In general, patients who were felt to be less fit, or to have excessive co-morbidity were selected for sequential CRT, or in some cases RT alone. In the diagnosis of stage III NSCLC, FDG-PET was routinely used since 2004 [in addition to computed tomography (CT) thorax and upper abdomen], and a magnetic resonance imaging (MRI) brain was standard since 2008. Advances in RT planning, which were implemented included: four-dimensional (4D) CT for treatment planning (in 2003); gated RT delivery for selected patients (in 2006); online image-guided radiotherapy (IGRT) (in 2007); and inverse-planned intensity-modulated radiotherapy (IMRT) treatment planning (in 2009) [Citation10–13]. The RT target volume was defined to incorporate the gross tumor and clinically involved/suspicious lymph nodes with motion [internal target volume (ITV) on 4D CT]. In addition the ipsilateral hilus was often included. The general approach for SEQ-CRT was to use the post-chemotherapy volume, but include pre- chemotherapy locations. For both CON- and SEQ-CRT the planning target volume (PTV) was defined as the ITV + 1cm. For CON-CRT, the planning-CT was typically performed directly after the first cycle of chemotherapy, and it was performed shortly after the final cycle for SEQ-CRT.
Data collection included: patient demographics [age, sex, WHO performance score, Charlson Comorbidity Index (CCI)]; tumor characteristics (staged using TNM 6th ed.); treatment details (CON-, SEQ-CRT, RT); RT-specific parameters (PTV in cm3), actual delivered RT dose (Gy). OS was determined using national mortality data retrieved from a National Record Database at 1 July 2012. Survival was measured from the start of RT since this date was available for all patients. For patients treated with CON-CRT, the RT typically began after one cycle of chemotherapy and was delivered concurrently with cycles 2 and 3 of full-dose platinum-based doublet chemotherapy, whereas for patients receiving SEQ-CRT, RT usually began after three cycles of chemotherapy. This means that in the case of those patients who started RT, OS for SEQ-CRT patients is systematically underestimated in comparison with the CON-CRT group by an amount that is in the order of 6–8 weeks. Minimum follow-up was 19 months. Early mortality was defined as patient death within five months after the start of RT (equal to approx. six months after the start of chemotherapy for patients receiving CON-CRT). For evaluation of trends, the following two-year time periods were assessed: 2003–2004, 2005–2006, 2007–2008 and 2009–2010. Analysis of OS with different PTV sizes was also performed. The cut-of point of 700 cm3 for PTV size also approximates to the mean volume identified in a prior publication from our group [Citation10], and we have also used this in a subsequent paper [Citation14]. In the present report, however, the analysis was also carried out for different a variety of PTV sizes.
Statistics
Median follow-up times were computed using the reverse Kaplan-Meier method. Patient and treatment characteristics were compared between CON- and SEQ-CRT groups using χ2-test for dichotomous variables, independent sample t-tests for normally distributed continuous variables and Mann-Whitney test for continuous variables that were not normally distributed. To test for differences between time periods we used χ2-tests, ANOVA and Kruskal-Wallis test. To test for trends over the time periods, we used the Mantel-Haenszel linear-by-linear association test, linear regression analysis and the Jonckheere-Terpstra test. OS was compared between groups using Kaplan-Meier analysis and the log-rank test. Independent risk factors for shorter OS were identified using Cox regression with forward selection. Predictors for early mortality (within five months after start of chemotherapy) were tested using logistic regression. Two-sided p-values < 0.05 were considered statistically significant. SPSS version 19.0 was used (IBM SPSS; New York, USA).
Results
A total of 1235 patients with a diagnosis of NSCLC were treated in the Department of Radiation Oncology between January 2003 and October 2010. Of these, 435 (35%) had stage III disease and 319 (73%) received radical intent non-surgical treatment consisting of RT, with or without chemotherapy. This group of 319 patients represented the study population. Of the remaining 116 patients, 74 (64%) had tri-modality therapy including surgery, 34 (29%) were treated with palliative intent, and eight (7%) had other treatments.
The characteristics of all 319 eligible patients are summarized in , together with characteristics of the 190 (60%) patients in the CON-CRT group and the 90 (28%) patients in the SEQ-CRT group. Two-thirds of the study population was male, and the mean age was 65 years (39–96). Fewer patients in the CON-CRT group were older than 70 years, compared to the SEQ-CRT group: 23% versus 34%, respectively (p = 0.05). Despite increases in absolute numbers over time, the proportion of patients with stage III NSCLC fell from 65% in 2003/4, to 28% in 2009/10 due to a large number of patients referred with lower stage disease. In 2003/4, 48/59 (81%) stage III patients received radical-intent non-surgical therapy, compared with 101/155 (65%) in 2009/10, when more patients received treatment schedules that included surgery.
Table I. Patient and treatment characteristics of all stage III NSCLC patients treated with curative non-surgical (chemo) radiotherapy (n = 319). Patients receiving concurrent (n = 190) and sequential chemo-radiotherapy (n = 90) are also shown separately.
About half of the patients (51%) had stage IIIA disease. Median PTV was 682 cm3 (range 56–3427 cm3). Median delivered RT dose for the total group was 59.8 Gy [inter-quartile range (IQR) = 55.0–66.0]. It was 60.0 Gy (IQR = 52.0–66.0) in the CON-CRT group compared to 59.8 Gy (IQR = 39.0–59.8) in the SEQ-CRT (p < 0.001). The significant difference is due to the left-skewed distribution in the CON-CRT group, with a large number of patients receiving at least 66 Gy (83/190) whereas the maximum dose in the SEQ-CRT group was 65 Gy. It is important to note that the comparison of absolute doses does not take into account the fact that patients in the RT and SEQ-CRT groups received different treatment schedules and fraction sizes with different biological effects (this is also dependent on the total dose). In the CON-CRT group, for example 90% of patients were treated using fraction sizes of 2 Gy, compared with the more potent 2.6 or 3 Gy per fraction received by 88% of SEQ-CRT patients. In the CON-CRT group, 66% (126/190) patients received a dose of at least 30 × 2 Gy, while 23% (43/190) received between 50 and < 60 Gy, in fractions of 2 or occasionally 1.8 Gy. In the SEQ-CRT group a total of 57% (51/90) patients received 22, 23 or 25 × 2.6 Gy and 30% (27/90) 13 or 15 × 3 Gy. In the CON-CRT group 90% (171/190) of patients completed the prescribed course compared with 93% (84/90) of the SEQ-CRT group (p = 0.361). The median OS for the 126 CON-CRT patients treated with at least 60 Gy, and for the 55 SEQ-CRT patients receiving at least 57.2 Gy, was 19.2 months [95% confidence interval (CI) 14.3–24.1] and 19.9 months (95% CI 12.6–27.2), respectively, (p = 0.803).
Patient and treatment characteristics are summarized separately for each of the four two-year time periods in . Mean age at inclusion did not differ significantly between quarters (p = 0.37). Although we found differences in the distribution of PTV sizes between the four periods (p = 0.011), there was no indication of a trend towards either larger or smaller volumes over the study period (p = 0.97). Over the study period significant trends were found towards a higher proportion of patients receiving CON-CRT and a smaller proportion receiving SEQ-CRT (p < 0.001 for both trends). shows the proportion of 319 eligible patients receiving RT, CON- and SEQ-CRT over the four two-year time periods. Over the study period there was a trend towards higher delivered radiation doses with median doses being 59.8 Gy (IQR = 39.0–59.8), 59.8 Gy (IQR = 47.0–59.8), 59.9 Gy (IQR = 50.0–66.0) and 66.0 Gy (IQR = 59.9–66.0) in 2003/4, 2005/6, 2007/8, and 2009/10, respectively. A trend towards higher doses was also found in the group treated with CON-CRT with median doses being 60.0 Gy (IQR = 50.0–60.0), 57.3 Gy (IQR = 50.0–60.0), 60.0 Gy (IQR = 50.0–66.0) and 66.0 Gy (IQR = 60.0–66.0) in 2003/4, 2005/6, 2007/8, and 2009/10, respectively.
Figure 1. Treatment of stage III non-small cell lung cancer in each two-year time period: concurrent chemo-radiotherapy (light grey dots), sequential chemo-radiotherapy (solid grey) and radical radiotherapy (white).
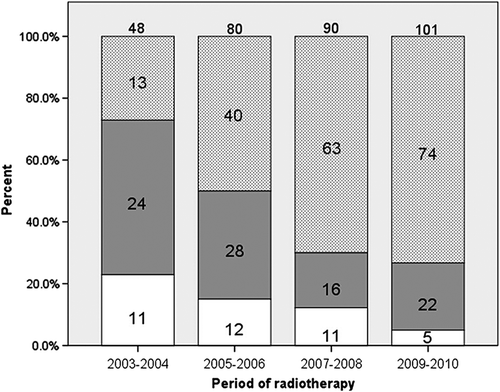
Table II. Differences in patient characteristics and overall survival between four time periods in the total group of stage III NSCLC patients treated with curative-intent non-surgical (chemo) radiotherapy (n = 319) and in the group treated with concurrent chemo-radiotherapy (n = 190).
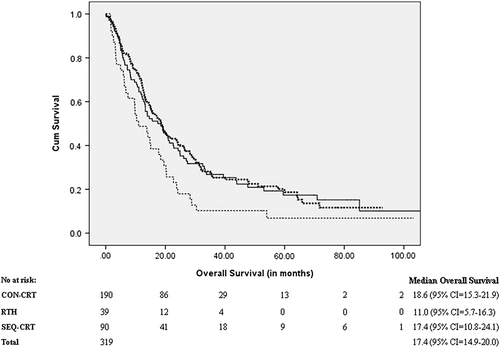
shows the OS for CON-, SEQ-CRT and RT groups. Median follow-up for the whole group was 57.2 months (95% CI 50.6–63.8). Median follow-up times were 49.1 months (95% CI 41.0–57.1) for the CON-CRT group and 73.5 months (95% CI 46.0–101.0) for the SEQ-CRT group. Median OS in the total group of 319 patients selected for the study was 17.4 months (95% CI 14.9–20.0). The median OS in the patient group treated with CON-CRT was 18.6 months (95% CI 15.3–21.9), in the SEQ-CRT group 17.4 months (95% CI 10.8–24.1) and in the RT group 11.0 months (95% CI 5.7–16.3). OS differed between the groups (log-rank test p = 0.024) with a post-hoc analysis revealing significant differences between the RT group and both the CON- and SEQ-CRT groups (p = 0.006 and p = 0.035, respectively), whereas OS was not significantly different between CON- and SEQ-CRT (p = 0.78).
Figure 2. Overall survival (OS) in stage III non-small cell lung cancer treated with concurrent chemo-radiotherapy CON-CRT (bold dotted line), radical radiotherapy RTH (dotted line) and sequential chemo-radiotherapy SEQ-CRT (unbroken line).
No differences were found in OS between the four time periods for the whole group (median OS: 2003/4 = 17.3 months; 2005/6 = 17.3 months; 2007/8 = 15.7 months; 2009/10 = 17.9 months, log-rank test p = 0.87), for the subgroup of patients treated with CON-CRT (median OS: 2003/4 = 18.9 months; 2005/6 = 13.2 months; 2007/8 = 18.6 months; 2009/10 = 19.5 months, log-rank test p = 0.96) and the subgroup of patients treated with SEQ-CRT (median OS: 2003/4 = 14.6 months; 2005/6 = 21.0 months; 2007/8 = 20.9 months; 2009/10 = 11.1 months, log-rank test p = 0.85).
Within the CON-CRT group, median OS for patients with good performance status PS = 0 according to WHO criteria (n = 47) was 32.0 months (95% CI 22.3–38.1), whereas median OS was 15.6 months (95% CI 11.8–19.4) for those patients (n = 142) with worse performance status PS ≥ 1 (log-rank test p = 0.02). Patients with smaller tumors (n = 102), which were defined as those with a PTV < 700 cm3, lived longer than the 88 patients with larger tumors (PTV > 700 cm3): median survival 24.3 months (95% CI 17.4–31.2) versus 13.7 months (95% CI 10.3–17.0), respectively (log-rank test p = 0.02). We found a significant relationship between PTV volume and OS (p = 0.01). For PTV < 350 cm3 (n = 17), 350–700 cm3 (n = 85), > 700–1050 cm3 (n = 52) and > 1050 cm3 (n = 36), median OS was 35.6 months (95% CI 0–71.3), 24.2 months (95% CI 18.3–30.2), 15.7 months (95% CI 10.5–20.9) and 10.3 months (95% CI 6.0–14.7), respectively. The sub-group 350–700 cm3 differed significantly from the groups 700–1050 cm3 and > 1050 cm3 (p = 0.039 and p = 0.002, respectively). This was not seen in the SEQ-CRT patients, perhaps due to smaller numbers in the PTV groups (n = 8, n = 37, n = 27 and n = 18, respectively).
OS did not differ between patients younger and older than 70 years or between patients (n = 32) with low-levels of co-morbidity (CCI = 0) and patients (n = 132) with more than one co-morbidity (CCI ≥ 1) with p-values for log-rank tests of 0.59 and 0.63, respectively. In multivariate Cox regression for OS in patients with PTV > 700 cm3, PS ≥ 1 and lower delivered dose were found to be independent risk factors (HR = 1.49, 95% CI 1.03–2.15, p = 0.035 for PTV > 700 cm3; HR = 1.91, 95% CI 1.23–2.97, p = 0.004 for PS ≥ 1; HR = 0.69, 95% CI 0.57–0.85, p ≤ 0.001 for 10 Gy increase in delivered dose).
Fifty-two (16%) patients of the total group died within five months of the start of RT (). In univariate logistic regression, early death was not found to be associated with gender, age or tumor-stage. No differences in occurrence of early death were found between treatment schedules. Early deaths were more common among patients with PTV-volumes exceeding 700 cm3 (OR = 2.68, 95% CI 1.43–5.02, p = 0.002).
Table III. Patient characteristics in the early mortality group (within 5 months of starting radiotherapy).
Discussion
Over successive two-year periods between 2003 and 2010, the absolute number of patients with NSCLC, including stage III disease, treated in our institution's radiation oncology department increased substantially. In the two years following the introduction of the 2004 national guidelines which recommended CON-CRT in selected patients, the proportion of patients with stage III NSCLC receiving radical non-surgical treatment with CON-CRT almost doubled, from 27% in 2003/2004 to 50% in 2005/2006. And by 2009/2010, this proportion had risen 2.5-fold to 73%. The increased use of CON-CRT was not associated with a detrimental effect on survival, and when benchmarked against a previous meta-analysis [Citation1], no excess in early-mortality was seen in patients treated with CON-CRT. This suggests the rapid and safe introduction of CON-CRT. Despite this, median OS with CON-CRT was no different from SEQ-CRT (even though survival was taken from the start of RT, which favors CON-CRT) and OS did not increase over time. This is consistent with the modest impact of changing treatment paradigms observed in the wider Dutch population [Citation15]. We confirmed that sub-groups of patients with WHO PS = 0 and those with planning target volumes < 700 cm3 who are treated with CON-CRT, may have a more favorable outcome [Citation10]. OS did not differ between younger and older patients, or between patients with low and higher levels of comorbidity. This is in line with an earlier report from our group [Citation10]. However, there is conflicting data on these important groups of patients (i.e. older patients and those with co-morbidity), and definitions may vary between studies [Citation16,Citation17].
The five-year survival rates in the present study, 17% and 19% for SEQ- and CON-CRT respectively, were also very similar and were higher than those reported by Auperin et al., 11% and 15%, respectively, in their meta-analysis of CRT studies conducted between 1988 and 2003 [Citation1]. This is even with a less selected patient population, as evidenced by the fact that 75% of CON-CRT patients with a recorded PS (189/190) had a score ≥ 1, compared with 48% in the meta-analysis [Citation1]. It is noteworthy therefore that in the meta-analysis, the four trials that most favored concurrent treatment had commencement rates for RT in the sequential arm as low as 64%, used a variety of RT dose/fractionation schedules (some of which would now be considered sub-optimal), and pre-dated the routine use of advanced technologies such as 4D CT or IGRT [Citation1]. We acknowledge that our analysis did not account for patients who dropped out of the intended CRT program, either during or after the chemotherapy phase. This could mean that only the fitter patients went on to receive RT in the SEQ-CRT group, and are likely to be represented in the present analysis. However, the concern that the CON-CRT group may therefore end up with more patients who might drop out during RT is not supported by our findings of similar RT completion rates for CON- and SEQ-CRT. We also note that both the CON- and SEQ-CRT groups contained similar numbers of patients receiving the highest RT dose levels (e.g. at least 30 × 2 Gy and 22 × 2.6 Gy, respectively). The results caution against over-stating the benefits of CON-CRT, and generalizing in a way that suggests that all CON- and SEQ-CRT is the same. It is reasonable to suppose that the institutional impact of CON-CRT may vary depending on the CON-CRT schedule and the SEQ-CRT program to which it is being compared.
The data in this study are from a period during which there was implementation and increasing use of new technologies such as daily online IGRT and IMRT. However, the impact of these technologies on patient outcome, and the impact of treatment planning techniques and doses to normal organs such as the heart and lung remains to be fully determined [Citation18]. The stable OS contrasts somewhat with the findings of Liao et al., who reported a substantial improvement in median survival in their institution after the introduction of 4D CT for treatment planning and IMRT [Citation19]. The difference might be that in the present series, use of 4D CT was routine throughout the study period. Although the median delivered RT dose in the present study increased by about 6 Gy over the study period, this was not temporally associated with longer OS. Possible reasons include limited power, too small an increase or the fact that this figure does not account for the higher potency of the combination of larger fraction sizes and relatively high total dose received by many patients undergoing SEQ-CRT.
Despite a critical approach to data collection, the primary limitation of this study is that it is retrospective with all the attendant risks, e.g. of bias and missing data. In addition, the lack of a denominator prevents ready comparison with other papers on this topic [Citation8,Citation9]. For this reason, the paper was restricted as far as possible to objective, quantifiable end-points that were the most reliable. We have already commented on the variation in chemotherapy start times between the SEQ- and CON-CRT groups. In addition, our report has focused on RT and has not evaluated the use of additional chemotherapy, or other systemic therapies, in greater detail (including on clinical progression). We also acknowledge the chance that patients seen in our institution could differ in some way from those seen in other regions or countries. Although toxicity and pattern of failure analysis would have been of interest, due to the retrospective character of this paper and the fact that much of the patient follow-up, including imaging was performed at other institutes, we chose to focus instead on better defined, more objective end-points. Finally, we have not, in this retrospective study, been able to tease out the relative contribution of various factors to the rapid change in pattern of care, including the dynamics within the multidisciplinary team and the availability of advanced RT technologies. Nonetheless, despite the limitations, the results are similar to the recent Quality Research in Radiation Oncology (QRRO) analysis from the USA, which found that the utilization of CON-CRT increased from 45% in 1998–1999 to 77% in 2006–2007, a figure similar to the utilization of CON-CRT in the third and fourth quarters of the present study [Citation20].
Conclusions
Between 2003 and 2010, CON-CRT for stage III NSCLC was rapidly and safely increased. However, OS did not increase and it was similar for both CON- and SEQ-CRT.
Declaration of interest: There has been no funding for this study. The Department of Radiotherapy, VU University Medical Center has Research Agreements with Varian Medical Systems Inc. and Brainlab AG, M. Dahele has received travel support and honoraria from Varian Medical Systems and Brainlab, W. F. A. R Verbakel has received speakers honoraria from Varian Medical Systems, B. J. Slotman has received travel support and honorarium from Varian Medical Systems and Brainlab and S. Senan has received speakers honoraria from Varian Medical Systems, and is also a member of the Trial Management Group for a phase III study in lung cancer sponsored by Lilly Oncology. P. M. van de Ven, E. J. F. van Reij, P. F. de Haan and E. F. Smit has reports conflicts of interest.
References
- Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90.
- Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103: 1452–60.
- Okawara G, Mackay JA, Evans WK, Ung YC, Lung cancer disease site group of cancer care Ontario’s program in evidence-based care. Management of unresected stage III non-small cell lung cancer: A systematic review. J Thorac Oncol 2006;1:377–93.
- O’Rourke N, Roqué I, Figuls M, Farré Bernadó N, Macbeth F. Concurrent chemo-radiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2010;6:CD002140.
- O’Rourke N, Macbeth F. Is concurrent chemoradiation the standard of care for locally advanced non-small cell lung cancer?. A review of guidelines and evidence. Clin Oncol 2010;22:347–55.
- Van Meerbeeck JP, Koning CC, Tjan-Heijnen VC, Boekema AG, Kaandorp CJ, Burgers JS. [Guideline on non-small cell lung carcinoma; staging and treatment]. Ned Tijdschr Geneeskd 2005;149:72–7.
- Non-small cell lung cancer. National guideline The Netherlands. Integraal Kankercentrum Nederland, 2011. [cited 2012 Dec 23]. Available from: www.oncoline.nl/niet-kleincellig-longcarcinoom.
- Wouters MW, Siesling S, Jansen-Landheer ML, Elferink MA, Belderbos J, Coebergh JW, et al. Variation in treatment and outcome in patients with non-small cell lung cancer by region, hospital type and volume in the Netherlands. Eur J Surg Oncol 2010;36:83–92.
- Vinod SK, Wai E, Alexander C, Tyldesley S, Murray N. Stage III non-small-cell lung cancer: Population-based patterns of treatment in British Columbia, Canada. J Thorac Oncol 2012;7:1155–63.
- Phernambucq EC, Spoelstra FO, Verbakel WF, Postmus PE, Melissant CF, Maassen van den Brink KI, et al. Outcomes of concurrent chemoradiotherapy in patients with stage III non-small-cell lung cancer and significant comorbidity. Ann Oncol 2011;22:132–8.
- Underberg RW, Lagerwaard FJ, Cuijpers JP, Slotman BJ, van Sörnsen de Koste JR, Senan S. Four-dimensional CT scans for treatment planning in stereotactic radiotherapy for stage I lung cancer. Int J Radiat Oncol Biol Phys 2004;60: 1283–90.
- Underberg RW, van Sörnsen de Koste JR, Lagerwaard FJ, Vincent A, Slotman BJ, Senan S. A dosimetric analysis of respiration-gated radiotherapy in patients with stage III lung cancer. Radiat Oncol 2006;1:8.
- Verbakel WF, van Reij E, Ladenius-Lischer I, Cuijpers JP, Slotman BJ, Senan S. Clinical application of a novel hybrid intensity-modulated radiotherapy technique for stage III lung cancer and dosimetric comparison with four other techniques. Int J Radiat Oncol Biol Phys 2012;83: 297–303.
- Wiersma TG, Dahele M, Verbakel WF, van de Ven PM, de Haan PF, Smit EF, et al. Concurrent chemoradiotherapy for large-volume locally-advanced non-small cell lung cancer. Lung Cancer 2013;80:62–7.
- Van der Drift MA, Karim-Kos HE, Siesling S, Groen HJ, Wouters MW, Coebergh JW, et al. Progress in standard of care therapy and modest survival benefits in the treatment of non-small cell lung cancer patients in the Netherlands in the last 20 years. J Thorac Oncol 2012;7: 291–8.
- Lee JH, Wu HG, Kim HJ, Kim DW, Lee SH, Kim TM, et al. Influence of comorbidities on the efficacy of radiotherapy with or without chemotherapy in elderly stage III non- small cell lung cancer patients. Cancer Res Treat 2012;44: 242–50.
- Kang KM, Jeong BK, Ha IB, Chai GY, Lee GW, Kim HG, et al. Concurrent chemoradiotherapy for elderly patients with stage III non-small cell lung cancer. Radiat Oncol J 2012;30:140–5.
- Cox JD. Are the results of RTOG 0617 mysterious?. Int J Radiat Oncol Biol Phys 2012;82:1042–4.
- Liao ZX, Komaki RR, Thames HD Jr, Liu HH, Tucker SL, Mohan R, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010; 76:775–81.
- Komaki R, Khalid N, Langer CJ, Spring Kong FM, Owen JB, Crozier CL, et al. Penetration of recommended procedures for lung cancer staging and management in the United States over 10 years: A quality research in radiation oncology survey. Int J Radiat Oncol Biol Phys 2013; 85:1082–9.
