Abstract
Purpose. The purpose was to examine characteristics, treatment and outcome in an unselected, prospectively registered complete population of patients with gastro-oesophageal adenocarcinoma cancer (GEA). Methods. All cases diagnosed with GEA between 2008 and 2009 in the Region of Southern Denmark (pop: 1 200 000) were registered. Patient characteristics, including performance status, stage and therapy, were retrieved from patient charts and used to compare sub-groups of patients. Results. Three hundred and thirty patients were registered as having GEA. Patients were divided into three clinical subgroups based on initial treatment option: group 1: patients with resectable GEA (n = 113); group 2: patients receiving first-line therapy (n = 107); group 3: patients receiving no tumour-directed therapy (n = 110). Median overall survival (95% CI) in the three groups was 36 months (25–not reached), 7.1 months (7–9) and 2.4 months (2–3), respectively. Seven percent of patients participated in clinical trials. Conclusion. Among patients not amendable to resection, around 30% are candidates for three-drug combination chemotherapy. Age > 65 years was found not to be a poor prognostic factor for survival, giving the possibility of treating elderly patients in the future. Many patients (approx. 30%), however, never received cancer-directed therapy. In order to improve survival in the entire population, it is important that future trials also focus on this group of patients.
Worldwide, cancers of the stomach and oesophagus are some of the most frequent types of malignancies and the second most common causes of cancer-related deaths. Together, they account for nearly 1.4 million new cases and 1.1 million deaths every year [Citation1,Citation2]. At the time of diagnosis, patients may be divided into three major treatment groups: patients with initially resectable disease, patients with locally advanced disease (T4NxM0) and patients with metastatic disease. The treatment decision is based on the above-mentioned stages as well as on histology, where the largest groups are adenocarcinomas and squamous cell carcinomas. Chau et al. [Citation3] evaluated patients with adenocarcinoma of the oesophagus, oesophagogastric junction or stomach (GEA) selected for first-line chemotherapy and found no differences in response rates and survival in relation to localisation of the primary tumour. Based on these findings, patients with locally advanced (lGEA) or metastatic disease (mGEA) are regarded and treated as a single entity. Previously, there was no standard treatment in patients with resectable disease (rGEA). In 2006, the MAGIC trial [Citation4] demonstrated the benefit of perioperative chemotherapy in patients with rGEA. Since 2008, it has been recommended, in Denmark, that patients with rGEA should be selected for perioperative chemotherapy independent of localisation [Citation5]. In recent years, trials have demonstrated the efficacy of first-line combination therapy in patients with lGEA and mGEA [Citation6–8], with an improvement in quality of life and a prolongation of median overall survival (mOS) from three to 8–10 months [Citation8]. Occasionally, it may be possible to perform a resection in patients with lGEA after initial down-sizing, and therefore a re-evaluation by a multidisciplinary team is important in selected cases. With a mOS of less than a year in lGEA and mGEA patients, knowledge about optimal treatment is urgently needed. Primarily information is obtained from randomised trials conducted in pre-specified highly selected patients, with the risk that only “the best” patients are evaluated. Little is known regarding the reproducibility of clinical trial results in the everyday clinical setting [Citation9,Citation10], and little is known about baseline characteristics and outcome in an unselected population. Information regarding the above-mentioned is important in order to evaluate the “true” benefit of therapy in different treatment groups, increase population-based survival and to plan future trials. The present study was planned to describe characteristics, treatment and outcome in an unselected cohort of patients diagnosed with GEA.
Material and methods
We prospectively registered all patients in the Region of Southern Denmark (population 1.2 million) during the period 1 January 2008 to 31 December 2009. The Region encompasses nine community hospitals and one university hospital, Odense University Hospital (OUH), where surgical, radiation and medical oncology is centralised. We established a database of all cases registered at OUH. To obtain data on the complete population, data from the Danish Cancer Registry (DCR) was retrieved using the unique 10-digit civil registration number all citizens use in contact with the health services [Citation11]. The two databases were compared, and dis-concordant cases were individually reviewed before inclusion, using patient charts and the available registry data.
We retrieved data on all patients with oesophageal, gastro-oesophageal junction and stomach cancer (C.15.0-16.9), regardless of histology. For the present analysis only patients with adenocarcinomas were included.
The registry was set up according to the Danish law for clinical research and complies with the Helsinki Declaration.
Clinical variables [performance status (PS), localisation of primary tumour and “up-front” treatment decision] were collected from patients’ medical charts. Blood test data were obtained prior to diagnosis for patients not receiving therapy, before surgery or neoadjuvant chemotherapy in resected patients, and before start of first-line therapy for patients treated at the Department of Oncology, OUH.
Statistical analysis
OS was estimated using the Kaplan-Meier method, with time counted from the date of first biopsy till death from any cause. Data cut-off was 1 October 2012. For comparisons between baseline characteristics, the Kruskal-Wallis test was used. Categorical variables were compared using a χ2-test. Patients were divided according to World Health Organization criteria into two groups: less than and more than 65 years of age. Comparison of survival curves was performed using a log-rank test. Confidence intervals (CI) are given at 95%, and the significance level was set to 0.05.
Results
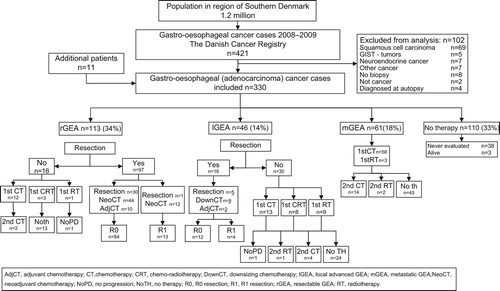
In the DCR, 421 were identified with primary malignancy of the stomach and oesophagus (registry no. C.150–C.16.9). We recorded 292 cases in our prospective registry OUH. Eleven cases were not reported in the DCR but included in our register after re-evaluation. Subsequently, 98 patients with non-adenocarcinoma histology and four patients diagnosed at autopsy were excluded, giving a total inclusion of 330 GEA patients (). Patients were distributed into four distinct groups (). One hundred and thirteen patients had rGEA, 46 patients had lGEA and 61 patients had mGEA. The fourth group of 110 patients did not receive cancer-directed therapy. In 65%, this decision was after an evaluation by a specialised surgeon or an oncologist, in the remainder the patient was not fit for an evaluation at a highly-specialised centre. The mOS for all patients was 9.5 months. For further analysis, patients were divided into three clinical sub-groups according to therapy given (); 1) 113 resected patients [rGEA (n = 97) and lGEA (n = 16)]; 2) 107 patients receiving first-line oncologic therapy (16 rGEA but not operable, 30 lGEA and 61 mGEA patients); and 3) 110 patients receiving no cancer-directed therapy.
Table I. Patient characteristic – by primary treament option.
Patient characteristics
Patient characteristics are given in , which shows that age differed significantly between all three groups (p = 0.0001). Resected patients and patients receiving first-line therapy were 14 and 10 years younger than patients not receiving cancer-directed therapy. Several baseline blood samples [haemoglobin, WBC, platelets, lactate dehydrogenase (LDH) and alkaline phosphatase (ALP)] differed significantly between groups. Resected patients had a higher level of haemoglobin and lower values of WBC, platelets, LDH and ALP than the other two groups.
Outcome in resected patients
Sixty-eight patients with oesophagus or gastro- oesophageal junction cancer had a transthoracic resection. Thirty-eight patients received a gastrectomy and five patients had a subtotal gastrectomy. An endoscopic resection was performed in two patients with a T1N0M0 tumour. Ninety-six patients had an R0 resection. Forty-four rGEA patients received perioperative chemotherapy and six lGEA patients received downsizing chemotherapy, most often with a three-drug combination of oxaliplatin, epirubicin and capecitabine (EOX) [Citation12]. Eleven patients with rGEA received adjuvant chemotherapy while one patient with lGEA received adjuvant chemotherapy. Thirty rGEA patients and four lGEA patients had a resection without perioperative or adjuvant chemotherapy. Two patients (2%) died within 30 days of the resection due to surgical complications. Recurrence was observed in 42 R0-resected patients. At the time of relapse, five patients had local recurrence, and it was possible to perform a subsequent curative treatment in four of these patients. The mOS in patients having a R0 resection was 39 months (95% CI 29–not reached) and the three-year OS was 49%. Forty-two patients (44%) are presently alive without sign of recurrence.
Seventeen patients had an R1 resection, and here mOS was 12.7 months (95% CI 8–33 months). Sixteen patients evaluated as rGEA at baseline did not have a resection. Reasons for not having a resection were medical co-morbidity (n = 9), disease progression during neoadjuvant chemotherapy (n = 5), patient choice (n = 2).
Outcome in patients selected for first-line therapy
The median age of patients selected for first-line therapy was 69.6 years. Most patients were diagnosed with metastatic disease and had a PS between 0 and 1 (69%) (). The triplet EOX regimen was used as standard therapy and 66 patients received this combination [Citation12]. Patients with rGEA or lGEA not suitable for a resection were evaluated for curative intended chemoradiotherapy (CRT). Eleven patients received CRT with a dose of 60 Gy/30 fractions and concomitant chemotherapy. Seventeen patients were found not to be candidates for standard three-drug chemotherapy and were offered a two-drug combination regimen with 5-fluorouracil (5-FU) and oxaliplatin. The reasons for this treatment decision were dysphagia and inability to swallow capecitabine (n = 13), co-morbidity (n = 2) and unknown (n = 2). Another 13 patients were not candidates for first-line chemotherapy and received radiotherapy (RT) with a palliative dose of 30 Gy/10 fractions. The distribution of first-line therapy is given in ().
Table II. Patients selected for first-line therapy.
At disease progression, 20 patients received second-line chemotherapy therapy. No therapy was recommended as a standard, but a second-line protocol was available. This protocol was established in cooperation with the National Health Programme, in which non-proven therapy can be offered to patients with advanced cancer, after approval by a Second Opinion Panel. This programme has had a major impact on the management of cancer patients in Denmark and has accelerated the introduction and implementation of new drugs. The therapy given was a combination of irinotecan and cetuximab every second week, and 13 patients participated in this trial [Citation13]. The mOS for all patients receiving first-line therapy were 7.1 months (95% CI 7–9). In patients selected for three-drug combination chemotherapy, the mOS was 7.8 (95% CI 6–10) months and 14.2 months in patients receiving CRT. In patients receiving two-drug chemotherapy and RT, mOS were 6.5 and 5.9 months, respectively.
Trial inclusion
During the registration period it was possible to participate in two trials evaluating first- and second-line chemotherapy. Twenty-three patients (7%) of the total cohort participated in clinical trials divided between 2% in the first-line setting and 5% in the second-line setting. Due to the limited numbers of patients participating in clinical trials, no statistical comparison has been made between patients selected for “standard therapy” and patients recruited into trials.
Outcome in patients receiving no cancer-directed therapy
One hundred and ten patients did not receive cancer-directed therapy. The median age was 79 years (range 41–95), and the main reasons for not receiving cancer-directed therapy were deterioration of health in (n = 84), co-morbidity (n = 10), patient wish (n = 8) and unknown (n = 8). The mOS was 2.4 months (95% CI 2–3).
Discussion
In a two-year period we registered all patients with a diagnosis of gastro-oesophageal adenocarcinoma in the Region of Southern Denmark and found a concordance with the DCR of 97% [Citation14]. The proportion of patients with pathological verification was 96%, which is comparable to the EUROCARE-4 program, with an average of 92% ranging from 56% to 98% [Citation15]. GEA was found to be a disease of elderly patients (median 69 years) and was often detected in advanced stages. In this unselected cohort, patients could be separated into three major groups of almost equal sizes; a resectable group, a group receiving chemotherapy or radiotherapy, and a group which never received cancer-directed therapy. Several studies have evaluated survival in a general population but few reported the distribution of patients into different treatment groups. Most studies predate the routine use of chemotherapy, for which reason only the number of resected patients is reported [Citation16–18]. A Dutch study reported outcome in 2212 patients from 1995–2006 [Citation19]. The resection rate was 29%, whereas 37% and 34%, of patients were selected for palliative treatment or no cancer-directed therapy, respectively. A SEER database reported outcome in 1356 GEA patients diagnosed in 2001 [Citation20]. Forty percent had resection, while 35% and 25% were treated with palliative therapy or no therapy, respectively. Patients older than 70 years were less likely to have therapy after adjusting for co-morbid conditions. Dividing our population by the age, we also found that younger patients < 65 years were significantly more likely to receive either a resection or first-line therapy than were older patients (p < 0.002).
In patients with rGEA, surgery alone is not sufficient. In Europe perioperative chemotherapy [Citation4,Citation21] is often recommended but neoadjuvant CRT [Citation22] or postoperative chemotherapy are other options. Since 2008, perioperative chemotherapy has been recommended by the Danish Gastric Cancer Group in patients with GEA stage T2-4N0 or T1-4N+ [Citation5,Citation23], and therefore patients in our cohort were not separated based on localisation of the primary. We found a promising three-year OS of 49% in patients having an R0 or an R1 resection, 66% of patients received perioperative chemotherapy. Perioperative chemotherapy was introduced in the daily practice by the time our study started, and this might explain why not all eligible patients were offered this modality. Ninety-six patients (85%) had a R0 resection. Patients with R0 resection had a significantly longer mOS (39 months) than patients having a R1 resection (12.7 months; p = 0.003).
In the first-line setting, a variety of different chemotherapy regimens have been investigated, but so far no treatment is accepted as a standard. There is a consensus that patients should be given platinum compound and 5-FU, but the potential benefit of a third drug (epirubicin or docetaxel) is still discussed [Citation8]. In our cohort, 66 patients received three-drug chemotherapy. Other treatment options were available based on physician and patient preferences. Survival benefit for any treatment strategy is subjected to major bias since patients were selected for treatment depending on baseline characteristics like co-morbidity and PS. A German publication covering the years 2006–2008 reported the choice of first-line chemotherapy in 1058 patients [Citation24]. There was a trend towards the use of more intense regimens over time. In 2009, 47% received a triplet regimen, while 47% and 6% received doublet or mono therapy. Concerning the use of therapy, the proportion of younger patients (< 65 years) receiving three-drug chemotherapy was almost twice as high as in the elderly patients (p = 0.0001). Patients in PS 0–1 were more likely to receive a triplet regimen than patients in PS > 1 (p = 0.001). Ten percent of the German patients participated in a clinical trial. In our smaller population, we found comparable results: median age (69 vs. 67 years), stage IV at diagnosis (69% vs. 62%), and PS 0–1 (69% vs. 73%), indicating that patients selected for first-line chemotherapy in a whole population do not differ between the two regions. However, we could not demonstrate that patients < 65 years selected for first-line therapy were more likely to receive three-drug chemotherapy than patients > 65 years (p = 0.35). Unfortunately, it was not possible to compare the impact of palliative therapy between the two populations because no outcome data are available in the German study.
When patients are exposed to “most optimal regimens” in phase III trials, mOS vary from 7.1 to 11.2 months [Citation6–8]. Shitara et al. evaluated baseline characteristics in GEA patients participating in randomised trials. The median age was 59 years and 83% had a PS between 0 and 1 [Citation25]. In our unselected cohort, patients receiving three-drug chemotherapy were 10 years older (median 69 years), 26% had a PS > 1 and the mOS of 7.8 months was slightly lower compared to results obtained in randomised trials. Interestingly, we did not find age to be a poor prognostic factor for survival (p = 0.83) in patients receiving three-drug chemotherapy, and we found no differences in the ability to complete planned therapy or receive second-line therapy in patients > 65 years.
Surprisingly, one-third of patients did not receive cancer-directed therapy. In general these patients were older (median 79 years), more patients were unstaged (28%) and the primary tumour was more frequently located in the stomach than in the oesophagus and gastroesophageal junction (p = 0.001). Therapy was primarily deselected due to poor general health. When patients are included in clinical trials, an expected overall survival of at least three months is often a general inclusion criterion. The observed mOS of only 2.4 months substantiate the decision not to offer chemotherapy. However, there was a trend towards a prolonged survival, 2.9 months vs. 2.1 months, in patients not evaluated by an oncologist. On the basis of the dismal survival, it is important that future studies focus on symptom relief with use of routine stent implantation in patients with dysphagia, palliative surgery, radiotherapy or “low-dose” chemotherapy. An evaluation by a multidisciplinary team might therefore also be important in this patient group in order to palliate symptoms and hopefully to prolong survival.
Conclusion
Our study reports staging, characteristics, therapy and outcome in a prospectively and unselected population of GEA patients. When assessing the impact of treatment, it is important to focus on the entire group of patients. Often survival rates are reported from either randomised trials, with the risk of evaluating only the “best patients”, or from population-based samples not reporting on treatment. The strength of the present study is its population-based nature and the fact that it reflects both treatment and survival in an unselected cohort. In non-resectable patients, only around 30% of a population are candidates for three-drug chemotherapy. The fact that age was found not to be a poor prognostic factor for survival or a barrier for receiving second-line therapy implies the possibility of treating a population of increasingly older patients and also to focus on this group of patients in future studies.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by a grant from the Danish Cancer Society.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clin 2011;61:69–90.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Cancer J Clin 2005;55:74–108.
- Chau I, Norman AR, Cunningham D, Oates J, Hawkins R, Iveson T, et al. The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma—individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol 2009;20:885–91.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med 2006;355:11–20.
- Available from: http://decv.gicancer.dk/Content/Files/Dokumenter/Kliniske%20Retningslinier%202011.pdf [2013 Apr 8].
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. New Engl J Med 2008;358:36–46.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006; 24:4991–7.
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database of Systematic Reviews [Internet]. 2010;3. Available from: http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD004064/frame.html. http://onlineibrary.wiley.com/store/10.1002/14651858.CD004064.pub3/asset/CD004064.pdf?v = 1&t = h6yvrakr&s = eb034c 73ad0b25d258e38c5d54d7fa1140ffb145. [Cited 2013 June 2]
- Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 2002;20:2109–17.
- Sorbye H, Pfeiffer P, Cavalli-Björkman N, Qvortrup C, Holsen MH, Wentzel-Larsen T, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 2009;115:4679–87.
- Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry – history, content, quality and use. Danish Med Bull 1997;44:535–9.
- Schonnemann KR, Jensen HA, Yilmaz M, Jensen BY, Larsen O, Pfeiffer P. Phase II study of short-time oxaliplatin, capecitabine and epirubicin (EXE) as first-line therapy in patients with non-resectable gastric cancer. Br J Cancer 2008;99:858–61.
- Schonnemann KR, Yilmaz M, Bjerregaard JK, Nielsen KM, Pfeiffer P. Phase II study of biweekly cetuximab in combination with irinotecan as second-line treatment in patients with platinum-resistant gastro-oesophageal cancer. Eur J Cancer 2012;48:510–7.
- Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the Cancer Registry of Norway: An overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31.
- De Angelis R, Francisci S, Baili P, Marchesi F, Roazzi P, Belot A, et al. The EUROCARE-4 database on cancer survival in Europe: Data standardisation, quality control and methods of statistical analysis. Eur J Cancer 2009;45: 909–30.
- Barchielli A, Amorosi A, Balzi D, Crocetti E, Nesi G. Long-term prognosis of gastric cancer in a European country: A population-based study in Florence (Italy). 10-year survival of cases diagnosed in 1985–1987. Eur J Cancer 2001;37:1674–80.
- Hansson L-E, Ekström A-M, Bergström R, Nyrén O. Surgery for stomach cancer in a defined Swedish population: Current practices and operative results. Eur J Surg 2000;166: 787–95.
- Lello E, Furnes B, Edna T-H. Short and long-term survival from gastric cancer. A population-based study from a county hospital during 25 years. Acta Oncol 2007;46:308–15.
- van de Poll-Franse LV, Lemmens VEPP, Roukema JA, Coebergh JWW, Nieuwenhuijzen GAP. Impact of concentration of oesophageal and gastric cardia cancer surgery on long-term population-based survival. Br J Surg 2011;98: 956–63.
- Cronin-Fenton DP, Mooney MM, Clegg LX, Harlan LC. Treatment and survival in a population-based sample of patients diagnosed with gastroesophageal adenocarcinoma. World J Gastroenterol 2008;14:3165–73.
- Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–21.
- van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, Henegouwen MIvB, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New Engl J Med 2012;366:2074–84.
- Sung Wook H, Dong Ho L, Sang Hyub L, Young Soo P, Jin Hyeok H, Jin Wook K, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol 2010;25:512–8.
- Hofheinz RD, Al-Batran SE, Ridwelski K, Gorg C, Wehle K, Birth M, et al. Population-based patterns of care in the first-line treatment of patients with advanced esophagogastric adenocarcinoma in Germany. Onkologie 2010; 33:512–8.
- Shitara K, Ikeda J, Kondo C, Takahari D, Ura T, Muro K, et al. Reporting patient characteristics and stratification factors in randomized trials of systemic chemotherapy for advanced gastric cancer. Gastric Cancer 2012;15: 137–43.