Abstract
Background. Metastatectomy in colorectal cancer (CRC) is now a standard of care with improved survival reported. Conversion chemotherapy has increased the population who are suitable for surgery. Here we assess patterns of care and treatment outcome in liver only metastases in South Australia using the clinical registry for advanced CRC. Methods. We analysed the outcomes for patients with liver only metastatic involvement from the SA Metastatic CRC Database with the aim to investigate the role of chemotherapy on liver resection and outcome in comparison to liver resection only and chemotherapy without liver resection. Patients who had no therapy or non-surgical liver interventions were excluded for this analysis. Results. One thousand nine hundred and eight patients were available for analysis, 687 (36%) had liver only metastatic disease and 455 (24%) had active therapy as defined. In total 54.2% (247/455) had chemotherapy alone, 19.1% (87/455) had liver resection alone, and 26.6% (121/455) had combined treatment. The three-year survival for chemotherapy, resection and combined treatment subgroups is 19.5%, 73.8% and 73.7%, respectively. The addition of chemotherapy to surgery did not improve survival. Switching chemotherapy was associated with a poorer outcome; three-year overall survival for chemotherapy switch was 62.5%, compared with same regimen pre- and post-74%, and chemo post-resection 80%. Conclusion. Liver only metastatic disease is common in CRC and patients undergoing liver resection have improved long-term survival. Survival for a combined approach of chemotherapy and hepatic resection is similar to surgery alone. Patients not suitable for surgery with liver only disease have a poorer prognosis highlighting the need for improved liver-directed therapies and attempts to covert non-resectable to resectable disease if possible.
For colorectal cancer patients with liver only metastatic disease, surgical resection offers the greatest likelihood of cure. In surgical case series, five-year survival rates post-resection average 40% [Citation1–4]. Patients with liver metastases that are resectable but left untreated have average survival of 6–12 months and rarely longer than two years [Citation5]. As a result of this significant survival advantage, when feasible, surgical resection is recommended.
Whilst there is a clear survival benefit from hepatic resection in patients with resectable liver metastases, the role of systemic chemotherapy following surgery for resectable disease is less defined. A pooled analysis of two phase III trials of post-operative 5FU chemotherapy following complete resection of colorectal liver metastases suggested there may be a benefit from adjuvant chemotherapy; [median overall survival (OS) of 62.2 months in the chemotherapy arm compared with 47.3 months in the surgery alone arm; HR 1.32; 95% CI 0.95–1.82; p = 0.095] [Citation6]. Peri-operative FOLFOX chemotherapy is, however, generally considered standard. This practice is based on the EORTC 40983 trial, which randomly assigned patients to either six cycles of FOLFOX4 before and six cycles after surgery, or surgery alone [Citation7]. This showed a non-statistically significant increase in median progression free survival in all randomly assigned patients from 11.7 to 18.7 months with chemotherapy. When patients who were unresectable were excluded from the analysis (17% in each arm), those who underwent surgery had a significantly higher three-year rate of PFS, 33.2% vs. 42.4% (HR 0.73; p = 0.025).
Downstaging chemotherapy in patients with initially unresectable metastases, has increased the proportion of patients for whom surgery is feasible. Patients with borderline resectable disease at presentation are often treated initially with systemic chemotherapy to decrease tumour bulk and exclude rapidly progressive disease. Patients who have tumours with aggressive biology, with widespread early disease progression, are unlikely to benefit from resection. If after initial treatment, the disease demonstrates response or stability, resection is more likely to improve outcomes although the optimal chemotherapy regimen has not been established. Adam et al. published a case series of 1104 patients with initially unresectable liver metastases who were given chemotherapy, following which 12.5% were converted to resectable [Citation8]. Similarly, in a French study of 330 patients, neoadjuvant FOLFOX allowed a 16% resection rate, and in those resected, a disease free survival of 36% after a median follow-up of 42 months [Citation9].
Given the different approaches used in the management of liver only metastatic disease, we sought to analyse treatment patterns outside of a clinical trial setting. Data from the South Australian Metastatic Colorectal Cancer Database registry was used to examine therapeutic decision making, with regard to choice of therapeutic agent, timing of chemotherapy delivery, and resection of liver metastases. In particular, we sought to analyse outcomes according to treatment group, to evaluate the benefit of resection over chemotherapy alone, and to examine whether the addition of chemotherapy to liver resection was associated with improved outcomes.
Methods
Data from the South Australian Metastatic Colorectal Cancer Database was used to investigate outcomes for patients with liver only metastatic disease. The establishment and maintenance of the database has been previously reported [Citation10]. In short the South Australian Clinical Registry for Metastatic Colorectal Cancer is managed by a committee composed of surgeons, oncologists, epidemiologists, clinical trials and data collection experts. The clinical membership is drawn from all of the major public hospitals (Flinders Medical Centre, Royal Adelaide Hospital and The Queen Elizabeth Hospital) and associated private hospitals. Representation from the Clinical Epidemiology Unit of the Department of Health and Cancer Control Research Centre of the Cancer Council South Australia in the capacity as experts in cancer registration and cancer epidemiology is also included. The statewide focus of the registry required the operational procedures of the project to be compliant with the South Australia Code of Fair Information Practice, Minimum Guidelines for Health Registries for Statistical and Research Purposes, and practice standards and protocols employed by the Department of Health Epidemiology Branch for operating the South Australian and Northern Territory Cancer Registries. The Registry was established with the authorisation under Section 64D of the South Australian Health Commission Act 6 which allows the collection of confidential data without the breach of law or any principals of ethics, protects the data from subpoena and imposes legal penalties for any unauthorised disclosure of the confidential information. Approval was granted by the South Australian Department of Health Human Research Ethics Committee and other individual institutional ethics committees where required. Data was collected over a five-year time period, from establishment of the database on 1 February 2006 through to a censor date of 14 February 2011. Median follow-up is 16.7 months. Data on patient characteristics, primary site, tumour biology, type of chemotherapy administered, resection margins, incidence of relapse and patterns of re-treatment were identified. The registry currently does not routinely collect size of lesions, number of lesions or CEA.
This information was ultimately used to examine the impact of chemotherapy and surgery on OS. Patients with liver only metastases were divided into three treatment-based groups: those who had chemotherapy only, those who underwent liver resection alone, and those who were managed with combined treatment, liver resection and chemotherapy. Combined treatment was defined as chemotherapy treatment administered within three months of liver surgery, either pre-operatively, peri-operatively or post-operatively. Disease-specific survival outcomes were analysed using Kaplan-Meier estimates, and χ2 were used for comparisons.
Results
From a total of 1908 patients with metastatic colorectal cancer, 687 (36%) had liver only metastatic disease. Four hundred and fifty-five patients with liver only disease ultimately had an intervention. Amongst these, 54.2% (247/455) had chemotherapy alone, 19.1% (87/455) had liver resection alone, and 26.6% (121/455) had combined treatment.
Patient characteristics
There were notable differences in the baseline characteristics of patients undergoing different treatment modalities (). Those who were treated with combined therapy, (chemotherapy and resection), were more likely to be younger than those treated with single modality therapy. Advancing age did not impact negatively on resection rates, with the median age of patients in the resection only cohort being 72 years. Male patients were more likely to be treated with resection, or resection and chemotherapy. A total of 59.2% (96/162) of female patients underwent chemotherapy only, and of patients treated with chemotherapy and resection, only 33.9% (41/121) were female.
Table I. Patient characteristics.
Tumour characteristics
Patients with colonic primaries were more commonly treated with chemotherapy or resection only, whereas patients with rectal primaries more often underwent chemotherapy as well as resection. In total 30.5% (139/455) of patients with liver only metastases had metachronous tumours. Ninety-four patients with metachronous tumours underwent resection. Of these, 54 had prior adjuvant therapy. These patients were more likely to undergo resection alone (33 patients) rather than chemotherapy and resection (21 patients).
KRAS mutation status was determined in a minority of each subgroup, although patients who received chemotherapy alone were more likely to have their KRAS mutation status established ().
Chemotherapy characteristics
In the subgroup treated with chemotherapy and liver resection, there was considerable variability in the timing of chemotherapy treatment. Chemotherapy was given pre-operatively to 33.9% (41/121) of patients, peri-operatively to 26.4% (32/121), and post-operatively to 37.2% (48/121). With respect to choice of chemotherapy regimen, oxaliplatin-based doublet chemotherapy was most common in all subgroups, 92.7% (38/41) pre-operatively treated patients, 90.6% (29/32) peri-operative, and 75% (36/48) post-operative. Amongst the patients treated peri-operatively, 31.3% (10/32) of those who commenced treatment with oxaliplatin-based doublet chemotherapy changed chemotherapy regimen post-operatively. These patients were then treated with irinotecan-based chemotherapy, and 5FU alone.
Resection characteristics
Surgical resection margins significantly differed between the subgroups treated with chemotherapy and surgery, and those managed with resection alone. In the group treated with surgery alone, clear margins were present in 86.2% (75/87) of resection specimens, compared with 95.9% (116/121) in the group treated with chemotherapy and surgery (p = 0.038). Surgical margins were not available for two patients in the resection alone group. Margins were involved in 11.5% (10/87) in the surgery only group, and 4.1% (5/121) in the combined treatment group.
Relapse and re-treatment
At the time of the analysis amongst patients in the group treated with resection only, 21.8% (19/87) patients have relapsed, and 14 of these have been subsequently treated with chemotherapy. Four of these patients have undergone re-resection. In the group treated with chemotherapy and liver resection, 26.4% (32/121) of patients have had disease recurrence. Of these, 17 have had further chemotherapy, seven re-resection, and eight combined treatment.
Survival
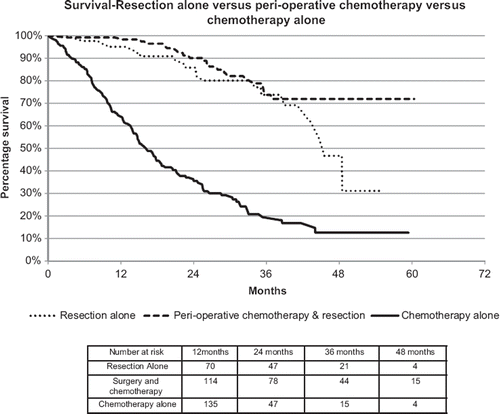
Survival is significantly better in those treated with resection (). The one-year survival rates for the chemotherapy, resection and combined treatment subgroups are 63.8%, 95.1% and 98.3%, respectively. The three-year survival rates indicate that this survival difference is maintained, with rates of 19.5%, 73.8% and 73.7%, respectively. A clear difference was seen between the groups treated with resection, and the group treated with chemotherapy alone. There is a difference in five-year survival between resection alone and chemotherapy/resection but this may reflect the relatively short follow-up at this stage. The addition of chemotherapy to surgery did not significantly improve survival, with no significant difference seen between the resection only and the resection with chemotherapy group.
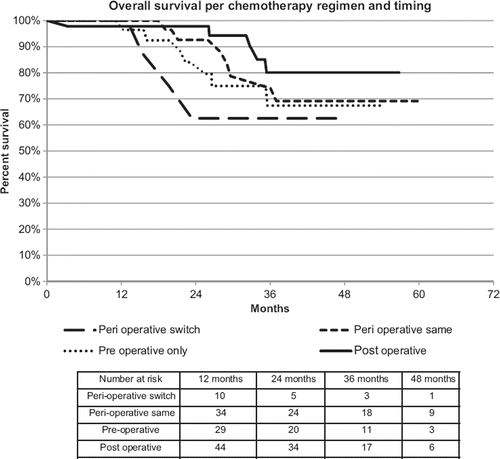
Patients who commenced pre-operative treatment with oxaliplatin-based chemotherapy and switched regimen post-operatively had poorer survival compared with those treated with the same regimen, and compared with those treated either pre-op or post-op only (see ). Three-year survival for those who underwent chemotherapy switch was 62.5%, compared with same regimen pre- and post-, 74% (p = 0.20), and chemo post-resection only, 80% (p = 0.038).
Discussion
Our study draws data from a statewide population-based registry of colorectal carcinoma. These patients were treated in tertiary referral centres and major private cancer centres of differing sizes across the state, and our analysis reflects real world practice. We used an epidemiological database to examine the outcomes of non-selected patients, and used survival as a measure of outcome. We were able to demonstrate a meaningful difference in survival between patients managed with chemotherapy alone and with resection alone as predicted. The addition of chemotherapy to resection did not show an improvement in survival in this data set, but there may have been differences between the groups which suggest that chemotherapy can salvage patients who would not otherwise have proceeded to surgery. Furthermore, chemotherapy had a positive impact on resection margins. In our study population, the timing of chemotherapy, number of cycles of treatment, and regimen administered varied considerably. The non-standardised nature of treatments may impact on our findings but does demonstrate that in a community setting there appears no standardised approach.
Our population-based survival rates for resection appear comparable with clinical trials of resected metastatic colorectal cancer. The three-year survival rates for chemotherapy, resection and combined treatment subgroups were 19.5%, 73.8% and 73.7%, respectively. A recent meta-analysis examining survival post-liver resection showed a three-year median survival of 57.5% across all patient groups. Data from 12 studies showed those treated with chemotherapy and resection had a median survival of 52.5% [Citation11]. The relatively shorter follow-up period may explain the difference at five years between the approaches and a follow-up analysis is planned.
Significant differences in survival were seen between patients who had peri-operative chemotherapy with the same chemotherapy regimen, and those who started with one regimen, and switched to another post-operatively (). Three-year OS was 74% and 80% years for those treated peri-operative FOLFOX and post-op FOLFOX, respectively compared to 62.5% with chemotherapy switch (p = 0.038 and 0.20, respectively). This may suggest that patients who switched treatments experienced a lack of response to pre-operative chemotherapy, and had associated inferior survival, although this was only statistically significant for post-operative alone versus peri-operative switch. Resection is still likely to be of value in this patient cohort, as the three-year OS of 62.5% remains favourable when compared with that of chemotherapy alone, which was associated with a three-year OS of 19.5% and median survival of 1.3 years. In contrast with the group of patients that switched chemotherapy, patients treated with chemotherapy pre-resection only, post-resection only or with the same regimen pre- and post-resection, appeared to have similar survival, irrespective of the timing of chemotherapy although of interest those who received only preoperative chemotherapy tracked lower at 67% three-year OS, again suggesting a difference in response and subsequent outcome in this group. One could hypothesise that this group did not respond to pre-operative chemotherapy or were not well enough to be considered for post-operative treatment.
Chemotherapy significantly impacted on liver resection margins. The addition of chemotherapy to surgery was strongly associated with clear margins, 86.2% versus 95.9% in the group treated with resection alone (p = 0.038). This finding is important, given the correlation between negative resection margins and improved survival [Citation12,Citation13]. Our survival curves after three years show marked separation between the resection only, and combined chemotherapy and surgery arms, in the later stages of follow-up. The accuracy of this separation may be supported by the observation that chemotherapy may improve resection margin outcome, and this may in turn offer improved longer term survival. Patients in the chemotherapy and resection arm have yet to meet median OS; analysis of survival at five years may lend further support to this observation which will occur with longer follow-up.
Our data does not distinguish between resectable patients treated with peri-operative chemotherapy as noted above, and those with initially unresectable disease, who underwent conversion chemotherapy and subsequent resection. Patients with extensive liver metastases are widely known to have poorer outcomes post-hepatic resection compared to those with limited disease [Citation14,Citation15]. Therefore, the overall prognosis of patients undergoing conversion chemotherapy is generally poorer. Grouping resectable and downstaged patients together in the combined arm may confound our results; the benefit of combined treatment compared with resection alone may be masked by the poorer overall prognosis of downstaged patients. More significantly though, our similar survival rates could indicate that chemotherapy allows downstaged patients to ultimately achieve a similar survival rate to primarily resectable patients.
In our study, patients with metachronous disease were more likely to undergo resection alone. This may reflect prior adjuvant chemotherapy or early detection of new liver lesions on monitoring scans, increasing the chance of resectability. In our study, 139 patients had relapsed disease. Of these, 94 underwent liver resection, either with or without chemotherapy. Within this group 54/94 (57.4%) had received prior adjuvant chemotherapy. These patients were more likely to be subsequently managed with resection alone, rather than further chemotherapy with resection (61% vs. 38%). This trend is likely to reflect clinician preference, and may be based on prior tolerance of chemotherapy, residual toxicity and patient inclination.
At the time of this analysis there were no patients who received an anti-EGFR with chemotherapy. This reflects the access to these drugs in Australia where they are limited to second and third line therapy. That said, anti-EGFR agents have not been reported to have a significant impact on resected stage II or stage III disease [Citation16] and thus may not be relevant in resectable liver disease where similarly the major target would be micro-metastatic disease. Down staging would be the major theoretical benefit given the reported higher RRs [Citation16,Citation17]. There are, however, few randomised studies examining the role of EGFR-targeted therapies in the management of resectable/potentially resectable liver metastases and the recently reported new EPOC trial of chemotherapy plus or minus cetuximab in this population supports ongoing research given the negative outcome in the combined arm (PFS chemotherapy/cetuximab 14.8 months vs. chemotherapy 24.2 months, p < 0.048) [Citation18]. The value of adding these agents to cytotoxic chemotherapy in this setting therefore remains uncertain.
Bevacizumab is the primary anti-angiogenic agent used in the management of advanced colorectal cancer. The role of this agent has been examined in the setting of conversion though given conflicting results the degree of benefit remains unclear. As with cetuximab, there is no evidence for benefit from bevacizumab in the adjuvant setting, which similarly may reflect the underlying biological differences between localised and metastatic disease. There is some evidence from select trials that bevacizumab plus cytotoxic chemotherapy provides high response rates in the potentially resectable setting, though this is not consistent [Citation19–23]. Thus there remains some debate as to whether the addition of bevacizumab improves the rate of conversion. Studies have shown however that bevacizumab use in the peri-operative setting is feasible from a toxicity aspect, with no significant impact on surgical complication rates, where bevacizumab was held during the peri-operative period [Citation24]. Further confirmatory randomised controlled trials are required before firm conclusions can be drawn.
In the treatment of resectable liver metastases with surgery, peri-operative FOLFOX chemotherapy is a generally accepted standard. OS data from an updated analysis of the EORTC 40983 trial was recently released after a median follow-up of 8.5 years. For all randomised patients, the five-year OS rate was 51.2% in the FOLFOX4 arm and 47.8% in the surgery alone arm, and for eligible patients these percentages were 52.4% and 48.3%, respectively. The difference in OS did not reach statistical significance (HR = 0.88, 95% CI 0.68–1.14, p = 0.34) (all randomized) [Citation25]. Ultimately this is the only positive trial for patients with resectable liver metastasis and would generally be accepted as the standard of care. Our results appear to suggest that in the real world practice, variations of this schedule are undertaken, with some clinicians electing for post-operative chemotherapy. The optimal timing of chemotherapy and surgery for liver confined metastatic disease is thus yet to be clearly defined and may be difficult as ongoing trails assessing this question are recruiting slowly.
Conclusion
Liver only metastatic disease occurs frequently in colorectal cancer. Liver resection offers these patients significant improvement in long-term survival. Patients with liver metastases for whom surgery is unsuitable have a poorer prognosis, and more often have synchronous metastatic disease from a colonic primary. Our population-based data has not shown a significant survival benefit of combined modality treatment with chemotherapy and hepatic resection on a population basis noting the limitations of this retrospective analysis. It has, however, shown an improvement in resection margins with chemotherapy. The optimal regimen and timing of chemotherapy is yet to be established, but is currently under investigation. Our study also illustrates the need to further explore treatment options for non-resectable liver only metastatic disease, given the poor survival for this group shown here.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–80.
- Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg 2008;247:125–35.
- Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with f-18 fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438–47; discussion 447–50.
- Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759–66.
- Wagner JS, Adson MA, Van Heerden JA, Adson MH, Ilstrup DM. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg 1984;199:502–8.
- Mitry E, Fields ALA, Bleiberg H, Labianca R, Portier G, Tu D, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. J Clin Oncol 2008;26: 4906–11.
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC intergroup trial 40983): A randomised controlled trial. Lancet 2008; 371:1007–16.
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg 2004;240:644–57; discussion 657–48.
- Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509–20; discussion 520–2.
- Neo EL, Beeke C, Price T, Maddern G, Karapetis C, Luke C, et al. South australian clinical registry for metastatic colorectal cancer. ANZ J Surg 2011;81:352–7.
- Gena PK, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin Epidemiol 2012:283–301.
- Cady B, Jenkins RL, Steele GD, Jr., Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: A critical and improvable determinant of outcome. Ann Surg 1998;227:566–71.
- Vandeweyer D, Neo EL, Chen JW, Maddern GJ, Wilson TG, Padbury RT. Influence of resection margin on survival in hepatic resections for colorectal liver metastases. HPB (Oxford) 2009;11:499–504.
- Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996;77:1254–62.
- Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: A contraindication to liver resection for multiple colorectal metastases?Ann Surg 2004;240:1052–61; discussion 1061–4.
- Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol 2011;22:1535–46.
- Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38–47.
- Primrose JN, Finch-Jones M, Valle JW, Sherlock D, Hornbuckle J, Gardner-Thorpe J, et al. A randomized clinical trial of chemotherapy compared to chemotherapy in combination with cetuximab in k-RAS wild-type patients with operable metastases from colorectal cancer: The new EPOC study. J Clin Oncol 2013;31.
- Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: No16966 updated results. Br J Cancer 2011;105:58–64.
- Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol 2011;22: 2042–8.
- Klinger M, Eipeldauer S, Hacker S, Herberger B, Tamandl D, Dorfmeister M, et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol 2009;35:515–20.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–9.
- Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol 2008;26:1830–5.
- Nordlinger B, Sorbye H, Glimelius B, Poston G, Schlag PM, Rougier P, et al. EORTC liver metastases intergroup randomized phase III study 40983: Long-term survival results. J Clin Oncol 2012;30.