Abstract
Three drug taxane-based regimens have shown activity in patients with metastatic or locally advanced gastric or gastro-esophageal cancer (GC/GEC). Limited tolerability of these regimens warrants treatment modification, particularly in regard of the proven equivalence of oxaliplatin and cisplatin as well as capecitabine and 5FU. Thus, a regimen with docetaxel (T), oxaliplatin (E) and capecitabine (X) was established and evaluated. Methods. Patients with metastatic or locally advanced GC/GEC, adequate organ function, ECOG PS 0–2 were enrolled. TEX regimen was administered as defined by the phase I trial with T 35 mg/m2 and E 70 mg/m2 on days (d) 1, 8 and X 800 mg/m2 bid on d 1–14 every 22 days. Primary endpoint was progression free survival (PFS) rate after 6 months. Results. Altogether 70 patients (15 phase I; 55 phase II) were eligible for analysis. Results of the phase II part were as follows: most common grade toxicities diarrhea (30%), nausea/vomiting and infections, PFS rate after 6 months 56.3%, response rate 43%, median PFS 6.9 and overall survival 13 months, respectively. Conclusion. The TEX regimen show similar efficacy compared to other infusional 5FU-based taxane and platinum containing triplets, but the reduced tolerability, in particular grade 3 diarrhea, limits the feasibility.
Gastric cancer is one of the most common causes of cancer-related death worldwide with about one million new cases and more than 700 000 deaths per year [Citation1]. Whereas gastric cancer mortality decreases in Europe, incidence and mortality rates for esophageal adenocarcinoma are increasing especially in Northern European countries, possibly related to gastro-esophageal reflux and increased body mass index [Citation2,Citation3]. Even 25 years after demonstrating the beneficial impact of chemotherapy compared to best supportive care (BSC) alone [Citation4–6], prognosis of locally advanced and metastatic gastric or gastro-esophageal cancer (GC/GEC) remains poor. Current standard chemotherapy regimens in the Western world consist of oral or intravenous (iv) fluoropyrimidines in combination with either oxaliplatin or cisplatin, alone or in combination with epirubicin (e.g. ECF regimen), docetaxel (e.g. DCF regimen), or trastuzumab for human epidermal growth factor receptor 2 (HER2) positive disease [Citation7–10]. The V325 trial showed significant superiority, including improved overall survival (OS) for the addition of docetaxel to a regimen of cisplatin and infusional 5-fluorouracil (5FU) [Citation7]. Although quality of life and time to deterioration of global health status significantly favored the triple regimen the proportion of patients withdrawing consent was doubled (11.6% vs. 21.7%) and the rate of grade 3 or 4 adverse events (AE) (59% vs. 69%) and complicated neutropenia (12% vs. 29%) were higher in the docetaxel-containing regimen [Citation11]. Equivalence of capecitabine and 5FU as well as oxaliplatin and cisplatin were shown in several phase III trials with a trend towards superior efficacy for either capecitabine- and/or oxaliplatin-based combinations with or without epirubicin [Citation8,Citation12,Citation13]. Moreover, superior OS favors capecitabine compared to 5FU, as demonstrated, e.g. in the combined analysis of two of these trials (REAL 2 and ML17032) (HR 0.87; 95% CI 0.77–0.98, p = 0.02) [Citation14]. In regard to the available data, modification of the active but less tolerable DCF regimen by fractionation of the docetaxel and platinum dose and substitution of cisplatin and 5FU by oxaliplatin and capecitabine seemed to be a meaningful approach. Therefore, a phase I trial for the determination of the maximum tolerated dose (MTD) of docetaxel, oxaliplatin and capecitabine (TEX regimen), and a subsequent phase II trial to evaluate the efficacy of this regimen was performed.
Patients and methods
Patient selection
Patients had to have a histo- or cytologically proven diagnosis of locally advanced, metastatic or recurrent adenocarcinoma of the stomach or the gastro-esophageal junction (GC/GEC) without curative intention, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, measurable disease according to response evaluation criteria in solid tumors (RECIST 1.0) (only phase II part), and adequate hematological, renal, and hepatic function defined by the following criteria: neutrophil count ≥ 1500/mm3, platelet count ≥ 100 000/mm3, creatinine-clearance ≥ 50 ml/min, total serum bilirubin less than or equal to the upper limit of the institutional normal range (ULN), and serum alkaline phosphatase ≤ 6 times ULN and transaminases ≤ 3.5 times ULN. Exclusion criteria were prior chemotherapy for metastatic disease; other active malignancies within the preceding year except for adequately treated basal cell cancer, squamous cell skin cancer, or in situ cervical cancer; clinical evidence of central nervous system metastases; clinically significant cardiovascular disease. All patients provided written informed consent before study entry according to the institutional regulations. The trial was approved by the institutional ethics boards and registered: NCT00511446.
Treatment administration
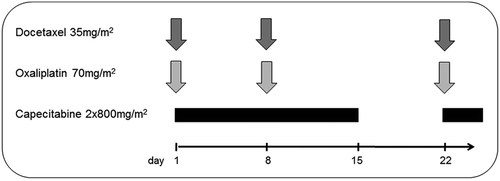
For the phase I dose-finding part, four dose levels were initially planned, using a schedule of administration for docetaxel (Taxotere; Sanofi-Aventis) and oxaliplatin (Eloxatin; Sanofi-Aventis) on days 1 and 8 and capecitabine (Xeloda; Roche) from days 1 to 14 of a 21-day treatment cycle (). The dose of oxaliplatin was kept at 70 mg/m2 iv on days 1 and 8, whereas dose of docetaxel was planned to be escalated from 35 to 40 mg/m2 iv on days 1 and 8, and for capecitabine from 800 to 1000 mg/m2 orally twice daily (bid) days 1–14.
In the phase II part, treatment was administered according to the dose level one with docetaxel 35 mg/m2 iv and oxaliplatin 70 mg/m2 iv, both on days 1 and 8, and capecitabine 800 mg/m2 orally bid days 1–14, determined as the MTD in the phase I part.
Dose limiting toxicities (phase I)
During the phase I part dose limiting toxicities (DLT) were defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI CTC AE v 3.0) as follows: 1) neutropenia grade 4 and fever (temperature > 38.5°C; 2) neutropenia grade 4 ≥ 7 days; 3) thrombocytopenia grade 4; 4) non-hematological toxicity ≥ grade 3 (apart from alopecia, nausea and vomiting); 5) deterioration of renal function (creatinine elevation) ≥ grade 2; 6) peripheral neuropathy ≥ grade 3; 7) hand-foot syndrome grade 3.
Dose adjustments (phase I/II)
A new treatment cycle was scheduled if the neutrophil count was ≥ 1500/mm3, platelet count was ≥ 100 000/mm3, and all relevant non-hematological toxic effects were grade 1 or lower (NCI CTC AE v 3.0). Dose reductions were based on the toxicity in the preceding cycle. The doses of all compounds were reduced by 25% for any grade 3 or 4 hematological toxicity (except anemia). Dose reduction of 25% for docetaxel and oxaliplatin was carried out for neuropathy lasting longer than seven days not interfering with function. In case of impairment of activities of daily living through neuropathy, docetaxel and oxaliplatin were permanently discontinued. Treatment was held for grade 3 non-hematological AE, excluding alopecia, nausea or vomiting, until resolution to grade 1 or lower, and resumed at a 25% reduction of doses of all three drugs, and discontinued for grade 4 non-hematological AE. In case of a drug-specific AE, e.g. hand-foot syndrome for capecitabine, solely the suspected drug was reduced. Capecitabine was reduced by 25% for mild renal impairment with a creatinine clearance from 30 to 50 ml/min. Patients requiring a treatment delay of more than two weeks due to toxicity or more than two dose reductions were removed from further treatment within the trial.
Study evaluations (phase I/II)
Pre-treatment evaluation included a complete medical history, physical examination, routine hematology and biochemistry analyses, and computed tomography (CT) scans of the abdomen and chest x-ray or optional CT scan of the thorax to define the extent of disease. Hematological (complete blood count) analyses were obtained at days 1 and 8, serum chemistry on day 1 in each cycle. Symptoms, physical examination results, performance status, and all adverse reactions were recorded before each treatment cycle according to NCI CTC AE v 3.0. Tumor assessment (CT scan) was performed every nine weeks (three cycles). Response rate was evaluated according to response evaluation criteria in solid tumors (RECIST) version 1.0 [Citation15]. If feasible, patients were discussed in a multidisciplinary team in order to evaluate potential secondary resectability.
Statistical methods
The phase I part was conducted to determine the MTD. The MTD was defined as the highest dose at which no more than 33% of patients experienced a DLT. Only DLTs that occurred during the first two cycles (six weeks) were considered in determining the MTD. Three patients were initially included in a cohort. Safety data were monitored real time. Dose escalation to the next level was planned if 0/3 patients experienced a DLT. If 1/3 patients experience a DLT, six more patients had to be treated on this dose level. As soon as the last patient of the cohort (either 3rd or 9th) reached day 42 (end of second cycle) safety data of all patients were reviewed for decision about moving to the next dose level or opening up a new cohort. To gain information about efficacy of the regimen a total of 12 patients were planned to be included in the MTD. No formal statistical assumption was performed for the continuation of development of the TEX regimen beyond the phase I part, although a cut-off point for disease control rate of at least 50% was determined.
The primary end point of the phase II part was progression free survival rate at 6 months (PFSR@6). A single-stage study design was planned. The primary goal was to detect evidence (with 80% power and one-sided type I error of 0.10) that patients on the TEX regimen had a median PFSR@6 of at least 55%, in contrast to an assumed reference value of 40% based on previously reported results with a fluoropyrimidine and platinum-containing regimen [Citation12,Citation13]. Under these assumptions the required sample size was 49 patients. With an estimated dropout rate of about 15%, 56 patients were included.
The intent-to-treat (ITT) population included all eligible patients, whereas the safety population included all patients receiving any chemotherapy. The Kaplan-Meier method was used to analyze the primary endpoint and censored event times. Confidence intervals of 90% were given for all calculated estimates. Baseline patient characteristics, response, and toxic effects were summarized in a descriptive way.
Results
Patients’ characteristics
In the phase I part 15 patients (six female and nine male) with a median age of 64 years (range 42–76) were included between July 2004 and September 2005. ECOG PS score of 0/1/2 was found in 33/53/14%, respectively. Four patients had prior gastrectomy, and all had metastatic disease (M1). All 15 patients received at least one treatment cycle and were thus included in the safety population. However, at the first dose level, one patient was removed due to a DLT within the first cycle, and two patients from the second dose level discontinued treatment after the first cycle.
Between August 2007 and March 2009, 56 patients were enrolled into the phase II part of the trial. One patient was found to be ineligible, after receiving treatment. Patients’ characteristics are summarized in . Twelve female and 43 male patients with a median age of 60 years (range 29–81 years) and ECOG PS score of 0/1/2 in 61/35/4%, respectively, were analyzed in the ITT population. Two patients did not receive study treatment, thus the safety population comprises 54 patients. The site of the primary tumor was stomach in 32 and GEJ in 22 patients (one missing), stage at baseline was locally advanced in nine and metastatic disease in 38 patients (eight missing). Prior resection of primary tumor was performed in 13 patients.
Table I. Patients` characteristics phase II part.
Treatment received
On the first dose level in the phase I part, three patients were initially included, of whom one patient had grade 4 bleeding from the primary tumor after the second administration (first cycle day 8) and discontinued further (study) treatment. Additional six patients were enrolled, without further DLTs (during the first two cycles). On second dose level, diarrhea grade 3 occurred as predefined DLT in 2/2 patients. Dose escalation was stopped and first dose level was determined as MTD. To reach 12 patients in MTD, four more patients were included without further DLTs. Median number of treatment cycles (21 days) was four (range 1–12).
A total of 302 cycles were administered in the phase II part, with a median number of six cycles per patient (range 1–17). Dose reductions were necessary in median two cycles per patient (range 0–8), and treatment was delayed in median one cycle per patient (range 0–6). Reasons for discontinuation of study treatment were progressive disease or death in 17 (31%), toxicity in 14 (25%), patients’ preference in three (5%), and secondary resection in eight patients (15%). Second line treatment was administered to 26 patients, mainly irinotecan combined with 5-fluorouracil. Two patients received trastuzumab as salvage treatment.
Efficacy
Within the 15 patients treated in the phase I part (ITT) five patients had a partial response (PR) and three had stable disease (SD), resulting in a disease control rate (DCR = PR+ SD) of 53.3%. Median PFS was 4.1 months (range 1.1–17.4) and median OS 9.8 months (range 1.1–18.7).
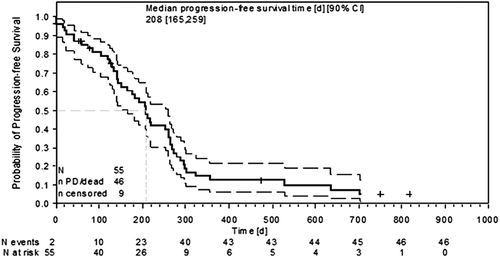
In the phase II part, 55 patients were included into the ITT analysis. Results are summarized in . PFSR@6 was 0.563 (90% CI 0.439–0.669). Thus, the primary endpoint of the phase II trial (PFSR@6 of at least 55%) was met. The overall response rate (ORR) was 43% with two complete (4%) and 21 PR (39%). PR could be confirmed (after nine weeks) in 15 patients. Twenty-one more patients (39%) had disease stabilization, resulting in a disease control rate of 82%. Median PFS was 6.9 months (90% CI 5.5–8.6) () and median OS 13 months, respectively (90% CI 9.5–16.8).
Table II. Efficacy according to RECIST 1.0 of phase II part.
Eight patients received secondary resection after achieving response to chemotherapy (one complete and one partial remission and six formally SDs) with median PFS of 10.3 and median OS of 26 months. Six patients had metastatic (liver: one, lung: one, distant lymph nodes: three and bones: one) and two locally advanced disease. Median number of cycles until resection was 4.5. All patients with metastases in distant organs (three) and one patient with distant lymph nodes recurred within median of five months after resection. Perioperative chemotherapy was administered in one patient with distant lymph node metastases receiving five cycles before and three cycles after secondary resection.
Toxicity
Treatment was generally well tolerated in an outpatient setting. During the phase I part, diarrhea grade 3 (according to NCI CTC AE version 3.0) occurred in 2/2 patients treated at dose level two. One case was accompanied by grade 3 febrile neutropenia and mucositis. At MTD, vomiting grade 3 occurred in two, nausea grade 3 in one, diarrhea grade 3 in two, anemia grade 3 in one, neutropenia grade 3 in three, and mucositis grade 3 in one of 12 patients, respectively. The mentioned toxicities occurred after the second cycle and were thus not counted as DLT. Most frequent all grade toxicities were diarrhea (66%), fatigue (58%), sensory neuropathy (58%), nausea (50%), alopecia (41%), mucositis (41%), and hand-foot syndrome (16%).
Main grade 3/4 AEs (phase II part) were diarrhea in 30% (only one patient with grade 4), infection (17%), nausea (13%) and vomiting (9%) of patients. AEs are summarized in . Diarrhea was the predominant grade 3–4 toxicity. In total, 20 severe adverse events (SAE) related to diarrhea or gastrointestinal toxicities were counted, with the majority (19) resolving. One patient, who was admitted for grade 3 treatment-related diarrhea developed fatal sepsis during the course of the SAE. However besides this case, diarrhea was well manageable with dose modifications. Rate of hematologic toxicity was rather low with neutropenia in 9% of patients (grade 3/4) and one case of febrile neutropenia. Altogether 70 SAEs were noted, with a median number of one per patient (range 0–6).
Table III. Toxicity according to National Cancer Institute Common Toxicity Criteria Version 3 of the phase II part.
Discussion
The addition of docetaxel to a combination regimen of platinum and fluoropyrimidine demonstrated a significant benefit in terms of efficacy and patient-related outcomes in a phase III trial, although toxicity was markedly increased [Citation7]. As shown in randomized trials without docetaxel, substitution of cisplatin by oxaliplatin and 5FU by capecitabine leads to (potentially) less toxic and more efficacious regimens. This multi-institutional phase I/II trial is the first report on the feasibility of a regimen with docetaxel, oxaliplatin and capecitabine in a large cohort of (GC/GEC) cancer patients. As a result of the dose-finding phase I part, a regimen with docetaxel 35 mg/m2 iv, oxaliplatin 70 mg/m2 iv, both on days 1 and 8, and capecitabine 800 mg/m2 orally bid days 1–14 was determined to be feasible. The efficacy data, with an ORR of 33% and a DCR of 53.3% in the 15 patients treated seemed worthwhile to be further evaluated in the phase II part of the trial. In the phase II part an ORR and DCR of 43% and 82%, respectively, and a median PFS of 6.9 and OS of 13 months was demonstrated. The primary endpoint was met and a clinically meaningful PFS rate at six months of 56.3% was shown.
These efficacy results are in line with other phase II trials with docetaxel, oxaliplatin and various fluoropyrimidine regimens, with reported ORR between 26 and 74%, median PFS 6.0–11 months and OS ranging from 8.0 to 15.5 months, although some are preliminary data published only in abstract form yet [Citation16–24]. Efficacy of these reports is summarized and compared to the current trial in .
Table IV. Comparison with other docetaxel, oxaliplatin and fluoropyrimidine containing first line regimens.
Early results of the GATE trial comparing docetaxel and oxaliplatin with or without 5FU or capecitabine randomizing 254 patients in three treatment arms suggest that the 5FU containing regimen is more efficacious than the capecitabine regimen in terms of ORR (46.6 vs. 25.6%), median time to progression (TTP) (7.7 vs. 5.6 months) and mOS (14.6 vs.11.3 months). However, lower doses per three week cycle in the GATE trial compared to the current trial for docetaxel (65 vs. 70 mg/m2), oxaliplatin (100 vs. 140 mg/m2), and capecitabine (625 vs. 800 mg/m2 bid) likely impacted efficacy [Citation21]. Similarly, other available three weekly schedules were using lower doses with docetaxel 50–75 mg/m2, oxaliplatin 100–130 mg/m2 and capecitabine 500–625 mg/m2 bid [Citation16,Citation22,Citation23]. The current TEX regimen is using the highest dosage for all three drugs reported yet, what may explain the reduced tolerability.
A potential benefit of a regimen with an oral fluoropyrimidine is the convenient administration, which was also feasible in the current TEX regimen in an outpatient setting. Grade 3/4 hematological toxicity, especially neutropenia (9%) was less frequent compared to other docetaxel containing triplets (FLOT: 48.1%, DCX: 76.5%, DCF: 82%). The most relevant grade 3/4 toxicity was mucosal, namely diarrhea occurring in 30% of patients (mainly grade 3). Notably, this was markedly higher as previously reported for 5FU-based triplets with docetaxel and oxaliplatin (FLOT: 14.8%, DCF: 19%) or cisplatin (DCX 11.8%) [Citation7,Citation17,Citation25]. However, largely variable rates of grade 3/4 diarrhea with other docetaxel, oxaliplatin, and capecitabine regimens have been noted ranging between 7% and 24% [Citation16,Citation21]. Although diarrhea was generally well manageable and did not result in permanent discontinuation of treatment in more than one patient, careful monitoring and early dose reduction have to be performed, if TEX regimen is used.
Conclusion
The combination of docetaxel, oxaliplatin, and capecitabine show comparable efficacy albeit reduced tolerability as reported from other triple chemotherapy regimens, including infusional 5FU or capecitabine, docetaxel and oxaliplatin (or cisplatin) in patients with locally advanced or metastatic GC/GEC. In regard to the relevant toxicity, particularly grade 3 diarrhea, the TEX regimen should currently not be used outside a clinical trial. The high response rate and long OS may indicate that this regimen may be suitable for relatively young patients with good performance status, and for patients with borderline resectable disease as a neoadjuvant treatment.
Acknowledgments
We want to thank all patients who participated in this trial, all institutions who included patients, and all the staff engaged in this study, especially the study team at the Koordinationszentrum Klinische Studien Halle.
Declaration of interest: The trial was supported by Roche and Sanofi-Aventis. The trial was supported by Roche and Sanofi -Aventis. AS, HJS and PCT-P received honoraria and research grants from Roche and Sanofi Aventis. MM received honoraria from Roche and Sanofi Aventis. RDH, DA received honoraria from Roche and Sanofi Aventis and research grants from Roche. The other authors have declared no conflicts of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78.
- Malvezzi M, Arfe A, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2011. Ann Oncol 2011;22:947–56.
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993;72:37–41.
- Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587–91.
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163–8.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006; 24:4991–7.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36–46.
- Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002; 20:1996–2004.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
- Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: The V-325 Study Group. J Clin Oncol 2007;25:3210–6.
- Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann Oncol 2009;20:666–73.
- Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435–42.
- Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: Evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 2009;20:1529–34.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
- Goel G, Jauhri M, Negi A, Aggarwal S. Feasibility study of docetaxel, oxaliplatin and capecitabine combination regimen in advanced gastric or gastroesophageal adenocarcinoma. Hematol Oncol Stem Cell Ther 2010;3:55–9.
- Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008; 19:1882–7.
- Shankaran V, Mulcahy MF, Hochster HS. Docetaxel, oxaliplatin, and 5-fluorouracil for the treatment of metastatic or unresectable gastric or gastroesophageal junction (GEJ) adenocarcinomas: Preliminary results of a phase II study. 2009Gastrointestinal Cancers Symposium 2009:Abstract No 47.
- Du C, Wang J, Huang J, Zhang D. Docetaxel plus FOLFOX4 as first-line therapy for advanced esophagogastric junction or gastric adenocarcinoma: A report of phase II study in a single center in China. 2010Gastrointestinal Cancers Symposium 2010:Abstract No: 94.
- Anderson EJ, Miner T, Mcnulty B. A phase II Brown University Oncology Group study of docetaxel, oxaliplatin, and capecitabine (DOC) for metastatic esophagogastric cancer. J Clin Oncol 2009;27(Suppl):abstr e15541.
- Van Cutsem E, Boni C, Tabernero J, Massuti B. Randomized phase II study (GATE study) of docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer. J Clin Oncol 2011; 29(Suppl):abstr 4018.
- Andersen M, Schonnemann KR, Yilmaz M, Jensen HA, Vestermark LW, Pfeiffer P. Phase I study of docetaxel, oxaliplatin and capecitabine (TEX) as first line therapy to patients with advanced gastro-oesophageal cancer. Acta Oncol 2010;49:1246–52.
- Liu Y, Ma T, Ye ZB, Zhang J, Zhu ZG. Efficacy and safety evaluation of docetaxel plus oxaliplatin and capecitabine in the treatment of advanced gastric adenocarcinoma: A single center non-controlled phase II clinical trial. Zhonghua Wei Chang Wai Ke Za Zhi 2010;13:177–80.
- Amarantidis K, Xenidis N, Chelis L, Chamalidou E, Dimopoulos P, Michailidis P, et al. Docetaxel plus oxaliplatin in combination with capecitabine as first-line treatment for advanced gastric cancer. Oncology 2011;80:359–65.
- Thuss-Patience PC, Hofheinz RD, Arnold D, Florschutz A, Daum S, Kretzschmar A, et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: A phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Ann Oncol 2012;23:2827–34.