Abstract
Background. To assess treatment tolerance by patients treated with a dose-adapted salvage radiotherapy (SRT) protocol based on an multiparametric endorectal magnetic resonance imaging (erMRI) failure definition model after radical prostatectomy (RP). Material and methods. A total of 171 prostate cancer patients recurring after RP undergoing erMRI before SRT were analyzed. A median dose of 64 Gy was delivered to the prostatic bed (PB) with, in addition, a boost of 10 Gy to the suspected relapse as visualized on erMRI in 131 patients (76.6%). Genitourinary (GU) and gastrointestinal (GI) toxicities were scored using the RTOG scale. Results. Grade ≥ 3 GU and GI acute toxicity were observed in three and zero patients, respectively. The four-year grade ≥ 2 and ≥ 3 late GU and GI toxicity-free survival rates (109 patients with at least two years of follow-up) were 83.9 ± 4.7% and 87.1 ± 4.2%, and 92.1 ± 3.6% and 97.5 ± 1.7%, respectively. Boost (p = 0.048) and grade ≥ 2 acute GU toxicity (p = 0.008) were independently correlated with grade ≥ 2 late GU toxicity on multivariate analysis. Conclusions. A dose-adapted, erMRI-based SRT approach treating the PB with a boost to the suspected local recurrence may potentially improve the therapeutic ratio by selecting patients that are most likely expected to benefit from SRT doses above 70 Gy as well as by reducing the size of the highest-dose target volume. Further prospective trials are needed to investigate the use of erMRI in SRT as well as the role of dose-adapted protocols and the best fractionation schedule.
Salvage radiotherapy (SRT) represents the mainstay curative treatment in patients with prostate cancer relapsing biochemically after radical prostatectomy (RP). In parallel to clinical prospective trials of radical radiotherapy (RT) for localized prostate cancer, biochemical control rates following SRT increase with dose as demonstrated by a recent systematic review of Ohri et al. [Citation1]. However, though higher SRT doses improve disease control, an increased risk of late genitourinary (GU) or gastrointestinal (GI) toxicity has to be expected with dose-escalated SRT regimens in a postoperative setting [Citation1].
A postoperative biochemical relapse is in more than 50% of the cases a surrogate of an underlying local relapse [Citation2], as the majority of failures after RP are located in the peri-anastomotic area (29–100% of the local recurrences [Citation2–4]) or around the bladder neck (16–40% [Citation2,Citation3]). The implementation of multiparametric endorectal-coil magnetic resonance imaging (erMRI) for SRT planning purposes has the potential to identify the suspected residual disease or the loco-regional recurrence and help to select those patients with an exclusive local relapse that may most benefit from SRT [Citation5].
In the present study, we have analyzed the acute and late toxicities in prostate cancer patients with biochemical relapse treated with a dose-adapted SRT protocol based on either the presence or the absence of local recurrent tumor after RP as defined by multiparametric erMRI studies.
Material and methods
From March 2001 through February 2010, a total of 177 consecutive prostate cancer patients relapsing after RP were treated with the same RT salvage treatment approach in two associated institutions. All patients underwent multiparametric erMRI studies [dynamic contrast-enhanced (DCE-MRI), spectroscopic imaging (MRSI) and diffusion-weighted MRI (DW-MRI) acquisitions with semiquantitative evaluation methods for DCE curves], detecting in 137 patients a local or loco-regional recurrence. Radiological criteria to define a suspicious local relapse were: masses with intermediate signal intensity on T2-weighted images enhancing after injection of contrast medium; region showing a fast enhancement on DCE-MRI images in the early phase followed by a plateau or washout; regions with low diffusion coefficients on DW-MRI sequences; choline to citrate ratio > 0.5 on MRSI images.
After exclusion of six patients with nodal relapse alone, a total of 171 patients, of which 131 with a suspected local relapse, were considered for this retrospective analysis. All patients were free of distant metastases. Patient demographics, disease and erMRI work-up characteristics are summarized in .
Table I. Patient demographics, disease and erMRI work-up characteristics (n = 171).
All patients were simulated with a full bladder using planning CT scans. From 2001 through 2004, the clinical target volume (CTVstandard) was defined by the anatomical limits of surgery from the seminal vesicles to the apex of the prostate, as proposed by the European Organization for Research and Treatment of Cancer (EORTC) trial 22911 on adjuvant RT [Citation6]. Later on, based on a study aiming to explore local patterns of failure after RP with erMRI, we shifted to a new CTV definition paradigm for adjuvant postoperative RT assumed to be able to cover for 95% of local failure possibilities in the prostatic bed (PB) region (CTVnew). Indeed, the CTVnew was defined by an approximately cylindrical shape of about 4 cm in height including the bladder neck and the penile bulb, centered roughly 5 mm posterior and 3 mm inferior the urethro-vesical anastomosis [Citation7]. figure 3 of the paper of Miralbell et al. illustrates the beam's-eye view figures showing the two CTV definition paradigms used in this study [Citation7].
In the 131 patients with suspicion of local failure on erMRI, a gross tumor volume (GTV) was defined and reported on the planning CT with the help of two expert uro-radiologists. The margins added to the CTV to create the planning target volume (PTV) were 10 mm, except posteriorly, towards the rectum, where a 6 mm margin was used. Field margins of 15–20 mm were used around the GTV to treat the boost volume with an additional dose of 10 Gy in 5 fractions after irradiation of the PB to 64 Gy in 1.8 to 2 Gy fractions. The dose was prescribed according to International Commission on Radiation Units & Measurements 50 guidelines, with the 95% isodose encompassing the PTV. Radiotherapy treatment characteristics are summarized in .
Table II. Radiotherapy treatment characteristics (n = 171).
Elective whole-pelvic RT (WPRT) was delivered at the discretion of the treating physician to 34 patients at high-risk of nodal disease based on the clinical (PSA) and histopathological (Gleason score, pT3b tumors) features. Patients receiving WPRT were more likely to present with a higher PSA value at salvage (mean 3.2 ± 3.8 ng/ml), with a pathologic Gleason score of ≥ 8 (n = 17, 50%), and/or with a seminal vesicles involvement (n = 18, 53%). In addition, neoadjuvant and/or concomitant androgen deprivation therapy (ADT) using luteinizing hormone-releasing hormone agonists was prescribed to 38 (22%) patients presenting one or more of the following high-risk features: a PSA value before salvage > 2 ng/ml, a pT3a or pT3b stage, and a Gleason score ≥ 8 in the pathologic specimen after RP. The median duration of ADT was 6.7 months (range, 3–34 months).
All patients were seen once-a-week while on treatment, six weeks after treatment completion, every three months during the first year of follow-up (FU), and every six months thereafter in the radiation oncology and/or in the urologic clinic. Acute and late (> 3 months after RT) GI and GU toxicity were graded on each visit according to the Radiation Therapy Oncology Group (RTOG) scoring system. Erectile dysfunction (ED) at baseline and at the last FU was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v. 3.0 scale. For late toxicity evaluation purposes, only patients with a minimum FU of 24 months after the end of salvage treatment (n = 109) were considered. The median FU of this cohort was 46.6 months (range, 24.6–96.3 months). Seventy-five percent of the 109 patients (n = 82) were treated with a boost. A digital rectal exam and a PSA dosage were performed at each visit and reported by the attending physician. A separated analysis on treatment outcome for the patients reported in this study will be object of further publication.
Comparisons were assessed using the χ2 and two-sample Student t-test for categorical and continuous variables, respectively. Actuarial late toxicity-free survival was estimated from the end of RT using the Kaplan-Meier method. Differences between groups were assessed with the log-rank test. Multivariate Cox proportional hazard regression analyses were implemented to evaluate the effect of different patient-, tumor- and treatment-factors influencing late GU and GI toxicity. Hazard ratios (HR), 95% confidence intervals (95% CI), and p-values were calculated. All statistical tests were two-sided and a p-value < 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS 17.0 statistic software package (SPSS Inc. Chicago, IL, USA).
Results
Acute toxicity
Among the 171 analyzed patients, the rate of acute grade 2 GU and GI toxicity at the end of salvage RT was 12.3% (n = 21) and 19.3% (n = 33), respectively. Three patients (1.8%) experienced an acute urinary obstruction (grade 4 GU toxicity) requiring temporary catheterization. No acute grade 3 or more GI toxicity was observed. Six weeks after treatment completion, 82.5% (n = 141) and 76.6% (n = 131) of patients were free of any GU and GI toxicity, respectively, while the corresponding rates were 51.5% (n = 88) and 30.4% (n = 52) at the end of salvage RT. Grade 2 GU and GI toxicity rates decreased in the six weeks following the end of RT to 4.1% (n = 7) and 2.9% (n = 5), respectively.
Late toxicity
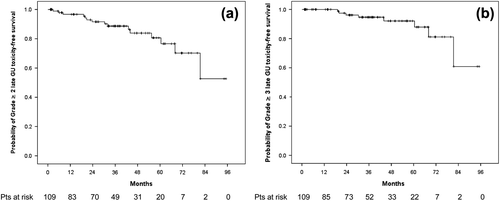
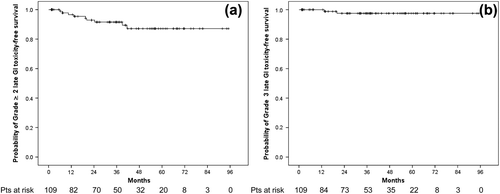
Late GU and GI toxicities for the 109 patients considered for the analysis are detailed in . For both GU and GI symptoms, grade ≥ 1 late toxicities were observed in 34.9% of patients.
Table III. Late genitourinary and gastrointestinal toxicities (RTOG scale): maximum score and toxicity at time of last follow-up (n = 109).
The four-year probability rate of grade ≥ 2 and ≥ 3 late GU toxicity-free survival was 83.9 ± 4.7% and 92.1 ± 3.6%, respectively (). Overall, seven patients (6.4%) experienced a grade 3 late GU toxicity. All but one patient with grade 3 late GU toxicity received a boost to 74 Gy. There were four patients presenting gross macrohematuria reversed after cauterization, while among three patients developing urethral strictures, two required elective endoscopic urethrotomy and one a permanent suprapubic urinary catheter placement after previous hyperbaric oxygen therapy. One patient treated to 74 Gy presented with grade 4 late GU toxicity 42 months after irradiation (combined symptomatic urethral stricture and contracted bladder requiring a cystectomy with concomitant pelvic lymphadenectomy in the context of an isolated regional failure post-SRT).
On multivariate analysis, the use of a boost (p = 0.048) and the presence of grade ≥ 2 acute GU toxicity (p = 0.008) were independently correlated with grade ≥ 2 late GU toxicity (). Although patients treated with CTVnew were more likely to receive a boost as compared to patients treated with CTVstandard (88% vs. 64.4%, p = 0.007), grade ≥ 3 late GU toxicity free-survival was not related with the CTV concept. Overall, only eight patients treated with IMRT and with a FU > 24 months were analyzed in this cohort. Of these patients, only one presented with grade 2 late GU toxicity. Actuarial rates for late GU toxicity were not different between patients treated with IMRT and 3D-CRT (p = 0.894). No impact in the four-year grade ≥ 2 late GU toxicity-free survival was observed for the interval time between RP to SRT of more or less than one year (83.3 ± 5.4% vs. 85.9 ± 9.3%, respectively, p = 0.691).
Table IV. Univariate and multivariate analysis of clinical, pathologic and treatment factors predicting for grade ≥ 2 late GU toxicity after SRT (n = 109).
The four-year probability rate of grade ≥ 2 and 3 late GI toxicity-free survival was 87.1 ± 4.2% and 97.5 ± 1.7%, respectively (). Two patients treated to 74 Gy (one treated to CTVnew and the second one to CTVstandard) presented with grade 3 late GI toxicity consisting of rectal bleeding reversed after cauterization. No patient experienced grade 4 late GI toxicity. At last FU, only two patients (1.8%) continued with grade 2 GI toxicity. In the univariate analysis, patients treated to 74 Gy with a boost showed a statistical trend for a lower four-year probability of grade ≥ 2 late GI toxicity-free survival as compared to patients treated to 64 Gy to the PB only (83.4 ± 5.3% vs. 100%, respectively, p = 0.093). Similar findings were observed in patients presenting with grade ≥ 2 acute GI toxicity at the end of SRT: a four-year probability of grade ≥ 2 late GI toxicity-free survival of 75.0 ± 11.5% compared to 91.0 ± 4.0% among patients with less than grade 2 acute GI toxicity (p = 0.095). No differences were observed for grade ≥ 2 late GI toxicity rates among patients treated using either CTVnew or CTVstandard (p = 0.809). In the Cox regression model, none of the covariates analyzed in the univariate analysis was significantly predictive of late grade ≥ 2 late GI toxicity.
At baseline, 11.9%, 18.3% and 58.7% of the 109 analyzed patients presented with grade 1, grade 2, and grade 3 ED, respectively. The rate of patients with grade 3 ED increased by roughly 11% after salvage treatment. At last FU, Grades 1, 2 and 3 ED accounted for 7.5%, 11.5% and 69.8% of patients, respectively.
Discussion
A linear correlation between radiotherapy dose, disease control and toxicity rates has been demonstrated in the salvage setting following biochemical recurrence of prostate cancer after curative RP [Citation1]. If every increment in PSA at salvage is associated to a lower probability of biochemical control after SRT [Citation1,Citation8], the delivery of higher SRT doses should be expected to improve disease control in these patients [Citation9]. However, the downside of dose escalation remains an increased risk of late GU and GI toxicity, estimated to be on the model proposed by Ohri et al. of 0.7% (95% CI 0.1–1.4%) and 1.2% (95% CI 0.3–2.1%) per Gy, respectively [Citation1].
If improvements in the therapeutic ratio of SRT may be obtained delivering the treatment at the lowest PSA level possible [Citation1], this study reported on the toxicity risk of an alternative treatment approach for patients relapsing after RP. Indeed, with the use of proper imaging able to detect the presence of local relapse in the tumor bed we wished to select a subset of patients harboring local disease only for which the use of dose escalation might help to improve disease control. Delivering 74 Gy to the suspected local recurrence in patients with positive erMRI, we escalated the SRT dose to the suspected tumor burden region only, avoiding delivering a potentially unnecessary high dose to the whole PB. However, in patients with no detectable local disease on erMRI studies we limited the dose to 64 Gy. This dose is considered by the American Society for Therapeutic Radiology and Oncology consensus guidelines able to achieve a durable biochemical control in a salvage setting [Citation10].
Long-term toxicity rates of previous published studies are illustrated on Supplementary Table I (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.837584) [Citation11–19]. Interestingly, in our series the rates of grade ≥ 3 late GU and GI toxicity (8.5% and 2.4%, respectively) were lower than expected on the basis of the model proposed by Ohri et al. for doses above 74 Gy using conventional radiotherapy techniques (i.e. 15% and 20% for GU and GI toxicity, respectively) [Citation1]. However, in analogy with other series delivering doses above 70 Gy [Citation12,Citation14,Citation16,Citation18], we observed that a relatively high percentage of patients (about 21%) treated with a boost developed grade ≥ 2 late GU side effects. Even if our 8.5% rate of grade 3 late GU toxicity in our boosted patients was inferior to the 14% four-year risk reported by Cozzarini et al. in patients treated to the whole PB to 72 Gy using 2D or 3D conformal techniques [Citation12], our findings suggest that a careful attention should be taken when dose escalated protocols are used in the salvage setting. Moreover, even if the notion that late GU toxicity increases even after 10 years post-RT is debated [Citation20], we acknowledge that a longer FU is probably needed to assess the real prevalence of long-term GU symptoms [Citation21].
In analogy with the series of Cozzarini et al., we found also the independent role of acute toxicity in predicting an increased risk of late grade 2 or greater late urinary side effects, suggesting a probably consequential component of late GU toxicity in the salvage setting post-RP [Citation12].
With a 93% of patients treated using 3D conformal techniques, we found a relatively high rate of grade ≥ 2 GI toxicity delivering 74 Gy as compared to other published series (Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.837584). We believe that the use of 3D-CRT techniques in most patients may probably explain our results. Indeed, in a series of Memorial Sloan-Kettering Cancer Center, IMRT resulted in an 8% reduction in late grade ≥ 2 GI toxicity compared to 3D-CRT, despite the larger average dose prescribed to patients treated with IMRT [Citation14]. It seems plausible that toxicity rates may be further reduced using high precision RT techniques in combination with image-guided systems [Citation22]. However, the observed 2.4% rate of more severe late GI toxicities (grade 3) with 74 Gy was only slightly superior to those reported by two recent published studies using IMRT for delivered doses of more than 70 Gy [Citation14,Citation18]. This result may be explained by the delivery of a focalized high-dose boost to the tumor recurrence only including a limited volume of rectal mucosa adjacent to the irradiated PB to doses above 70 Gy.
Although the CTVnew paradigm was expected to reduce the irradiation of normal tissues nearby [Citation7] in this series we were unable to demonstrate a clear improvement in toxicity rates with the new model of target definition as compared to patients treated to the CTVstandard. It is possible, however, that this new CTV paradigm may help to optimize the dose distribution and improve treatment tolerance particularly when high doses are delivered to the whole PB and not only to the gross recurrence. The impact of such a CTV proposal on clinical outcome remains to be determined.
Some limitations of this study need to be addressed. First, the retrospective nature of this study analyzing a small population of patients treated over 10 years with the same protocol in two affiliated institutions, with some inherent inhomogeneities in patient selection and treatment schedules, such as the use of WPRT and concomitant ADT in approximately one fifth of the patients. Second, we acknowledge that the use of the RTOG toxicity scoring scale to assess acute and late toxicity may have underreported toxicity events such as rectal and/or urinary urgency or incontinence. Moreover, we acknowledge the inherent pitfalls related to the inaccuracy in the definition of the GTV, as images of local failure were translated from MRI studies (with an endorectal coil) to planning CTs (without endorectal probe), as well as to the lack of histo-pathological confirmation of the suspected local relapse as detected by erMRI studies. However, the relatively high percentage of positive findings on erMRI studies observed in this series may be explained by the use of multiparametric acquisitions with an endorectal coil, interpreted by a stable team of experienced uro-radiologists. Previous published works have demonstrated the high reliability of multiparametric erMRI, in detecting local recurrence after RP, even in patients with PSA levels of less than 1.5 ng/ml [Citation5]. This is particular true when the combined modality of MRSI and DCE-MRI is used as in our series. For a PSA value at salvage similar to ours (1.26 and 1.9 ng/ml, respectively, vs. 1.89 ng/ml in our series), two recent studies showed for the combination of MRSI and DCE-MRI a very high diagnostic accuracy, with a sensitivity of 87% and 94% and a specificity of 94% and 100%, respectively [Citation23,Citation24].
In conclusion, the dose-escalated treatment approach investigated in this study may serve as an hypothesis generating basis for further prospective trials aiming to improve the therapeutic ratio in the salvage setting. The role of erMRI for selecting patients that are most likely expected to benefit from SRT doses above 70 Gy as well as for limiting the potential side effects of dose-escalated SRT protocols by reducing the size of the highest dose target volume needs to be prospectively evaluated.
Supplementary Table I
Download PDF (137.4 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ohri N, Dicker AP, Trabulsi EJ, Showalter TN. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer 2012;48:837–44.
- Connolly JA, Shinohara K, Presti JC, Jr. Carroll PR. Local recurrence after radical prostatectomy: Characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology 1996;47:225–31.
- Sella T, Schwartz LH, Swindle PW, Onyebuchi CN, Scardino PT, Scher HI, et al. Suspected local recurrence after radical prostatectomy: Endorectal coil MR imaging. Radiology 2004;231:379–85.
- Silverman JM, Krebs TL. MR imaging evaluation with a transrectal surface coil of local recurrence of prostatic cancer in men who have undergone radical prostatectomy. AJR Am J Roentgenol 1997;168:379–85.
- Alfarone A, Panebianco V, Schillaci O, Salciccia S, Cattarino S, Mariotti G, et al. Comparative analysis of multiparametric magnetic resonance and PET-CT in the management of local recurrence after radical prostatectomy for prostate cancer. Crit Rev Oncol Hematol 2012;84:109–21.
- Davis JB, Reiner B, Dusserre A, Giraud JY, Bolla M. Quality assurance of the EORTC trial 22911. A phase III study of post-operative external radiotherapy in pathological stage T3N0 prostatic carcinoma: The dummy run. Radiother Oncol 2002;64:65–73.
- Miralbell R, Vees H, Lozano J, Khan H, Molla M, Hidalgo A, et al. Endorectal MRI assessment of local relapse after surgery for prostate cancer: A model to define treatment field guidelines for adjuvant radiotherapy in patients at high risk for local failure. Int J Radiat Oncol Biol Phys 2007; 67:356–61.
- King CR. Adjuvant radiotherapy after prostatectomy: Does waiting for a detectable prostate-specific antigen level make sense?Int J Radiat Oncol Biol Phys 2011;80:1–3.
- King CR, Kapp DS. Radiotherapy after prostatectomy: Is the evidence for dose escalation out there?Int J Radiat Oncol Biol Phys 2008;71:346–50.
- Cox JD, Gallagher MJ, Hammond EH, Kaplan RS, Schellhammer PF. Consensus statements on radiation therapy of prostate cancer: Guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate- specific antigen levels after radical prostatectomy. J Clin Oncol 1999;17:1155.
- Chawla AK, Thakral HK, Zietman AL, Shipley WU. Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: Analysis of efficacy and prognostic factors. Urology 2002;59:726–31.
- Cozzarini C, Fiorino C, Da Pozzo LF, Alongi F, Berardi G, Bolognesi A, et al. Clinical factors predicting late severe urinary toxicity after postoperative radiotherapy for prostate carcinoma: A single-institute analysis of 742 patients. Int J Radiat Oncol Biol Phys 2012;82:191–9.
- Feng M, Hanlon AL, Pisansky TM, Kuban D, Catton CN, Michalski JM, et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1417–23.
- Goenka A, Magsanoc JM, Pei X, Schechter M, Kollmeier M, Cox B, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol 2011;60:1142–8.
- Jereczek-Fossa BA, Zerini D, Vavassori A, Fodor C, Santoro L, Minissale A, et al. Sooner or later? Outcome analysis of 431 prostate cancer patients treated with postoperative or salvage radiotherapy. Int J Radiat Oncol Biol Phys 2009;74:115–25.
- Kruser TJ, Jarrard DF, Graf AK, Hedican SP, Paolone DR, Wegenke JD, et al. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer 2011;117:2629–36.
- Neuhof D, Hentschel T, Bischof M, Sroka-Perez G, Hohenfellner M, Debus J. Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys 2007;67:1411–7.
- Ost P, Lumen N, Goessaert AS, Fonteyne V, De Troyer B, Jacobs F, et al. High-dose salvage intensity-modulated radiotherapy with or without androgen deprivation after radical prostatectomy for rising or persisting prostate-specific antigen: 5-year results. Eur Urol 2011;60:842–9.
- Wiegel T, Lohm G, Bottke D, Hocht S, Miller K, Siegmann A, et al. Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome – results of a retrospective study. Int J Radiat Oncol Biol Phys 2009;73:1009–16.
- Ghadjar P, Jackson A, Spratt DE, Oh JH, Munck AF, Rosenschold P, Kollmeier M, et al. Patterns and predictors of amelioration of genitourinary toxicity after high-dose intensity-modulated radiation therapy for localized prostate cancer: Implications for defining postradiotherapy urinary toxicity. Eur Urol Epub 2013 Feb 4.
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9.
- Ost P, De Gersem W, De Potter B, Fonteyne V, De Neve W, De Meerleer G. A comparison of the acute toxicity profile between two-dimensional and three-dimensional image-guided radiotherapy for postoperative prostate cancer. Clin Oncol 2011;23:344–9.
- Panebianco V, Sciarra A, Lisi D, Galati F, Buonocore V, Catalano C, et al. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP). Eur J Radiol 2012;81:700–8.
- Sciarra A, Panebianco V, Salciccia S, Osimani M, Lisi D, Ciccariello M, et al. Role of dynamic contrast-enhanced magnetic resonance (MR) imaging and proton MR spectroscopic imaging in the detection of local recurrence after radical prostatectomy for prostate cancer. Eur Urol 2008;54:589–600.