Abstract
Background. There is limited knowledge regarding progressive resistance training during adjuvant chemotherapy and the risk of developing breast cancer-related lymphedema (BCRL). Furthermore, no studies have investigated the safety of resistance training with heavy loads (> 80% 1 repetition maximum) in this population. ‘Body and Cancer’ is a six-week, nine-hour weekly, supervised, multimodal exercise intervention utilizing progressive resistance training with heavy loads for cancer patients undergoing chemotherapy. The purpose of the present study was to estimate the prevalence of BCRL in former participants, and identify associations between progressive resistance training with heavy loads, and the development of BCRL. Material and methods. This was a descriptive study. Population: Women treated for breast cancer (n = 149), who had participated in the ‘Body and Cancer’ exercise intervention between 1 January 2010 and 31 December 2011 participated in a structured telephone interview. The average follow-up time was 14 months (range 4–26). A clinical diagnosis of BCRL reported by the participant was the primary outcome. Results. A total of 27.5% reported that they had been diagnosed with BCRL by a clinician. This was true for 44.4% with axillary node dissection. No statistically significant association between strength gains during the exercise intervention, and the development of BCRL was observed, nor was self-reported participation in progressive resistance training with heavy loads up to three months post-intervention. Conclusion. The prevalence of BCRL among former “Body and Cancer” participants at follow-up was 27.5%. There appears to be no association between performing heavy resistance training during adjuvant treatment (chemotherapy/radiotherapy), and the development of BCRL. However randomized controlled trials should be performed to confirm this observation.
Breast cancer-related lymphedema (BCRL) as a result of acquired interruption or damage to the axillary lymphatic system is associated with significant physical, functional, and psychosocial burden [Citation1]. The incidence and prevalence of BCRL have been difficult to quantify due to a lack of a standardized measurement method and a uniform definition of what constitutes BCRL, as well as the lack of an evidence-based definition of transient versus chronic lymphedema [Citation1–3]. Moreover, prevalence rates have been found to vary based on the surgery performed and the extent of adjuvant treatment ranging from 13% to 65% [Citation4]. In a meta-analysis from 2013, Disipio et al. found a pooled estimate for incidence of 16.6% based on 72 studies [Citation1]. However, incidence rates varied depending on study design ranging from 8.4% in retrospective cohort studies to 21.4% in prospective cohort studies, as well as a result of diagnostic method ranging from 5.0% with lymphoscintigraphy to 28.2% when multiple measurement methods were applied. The incidence of BCRL seemed to increase with time up to two years from diagnosis or surgery (12–< 24 months, 18.9%), after which a decrease in incidence was observed. Lastly, the incidence of BCRL was four times higher among women who had AND (19.9%) than in women who had sentinel node biopsy (SNB) (5.6%).
There has been a longstanding concern that progressive resistance training (PRT) increased the risk of developing BCRL [Citation5]. However, a growing body of evidence indicates that PRT does not increase BCRL risk [Citation5,Citation6]. Furthermore, it is well demonstrated in the literature that PRT has a beneficial effect on a number of the side- or late effects related to breast cancer treatment by positively impacting self-perceptions of body image [Citation7], increasing vitality [Citation8], lean body mass [Citation9,Citation10], bone mineral density [Citation11], and muscular strength [Citation9,Citation10,Citation12]. Indeed, 2010 guidelines from the American College of Sports Medicine (ACSM) [Citation6] advocate PRT. However, none of the seven studies that these guidelines are based on were conducted on patients undergoing adjuvant treatment (chemotherapy and/or radiotherapy). Furthermore, the heaviest loads lifted corresponded to three sets of 10 repetitions [Citation12,Citation13], considered moderate resistance [Citation14]. Since the ACSM guidelines were published, four randomized controlled exercise trials utilizing PRT during adjuvant treatment have been performed [Citation9,Citation15–17]. Only one of these had BCRL as the primary outcome. Furthermore, maximum loads of 60–70% of 1 RM (moderate load) were the heaviest loads lifted. Recently, Cormie et al. conducted two studies which examined the safety of heavy resistance training in women with BCRL. The studies found that resistance training with heavy loads (> 80% 1 RM) did not acutely exacerbate an existing lymphedema [Citation18], and was found to be a safe training mode, associated with improvements in physical function and quality of life [Citation19]. However, as these studies were performed in women with BCRL, a gap in knowledge exists concerning the safety of heavy load PRT in regard to BCRL risk. Therefore studies are needed that investigate the safety of PRT during adjuvant treatment with BCRL as the primary outcome, as well as PRT with heavier loads.
Originally a RCT (for details see Adamsen et al. [Citation20]) ‘Body and Cancer’ (B&C) has been offered as an exercise intervention for cancer patients undergoing chemotherapy in the Copenhagen area since 2007. To date approximately 1300 participants representing over 21 diagnoses have participated in this six-week, nine-hour weekly, supervised multimodal exercise intervention. Among the unique characteristics of this intervention is the utilization of low intensity components (relaxation- and body awareness training and massage) with high intensity components (aerobic- and resistance training).
Of interest for the present study are the high intensity days (Monday, Wednesday, Friday) where participants engage in a cardiovascular warm-up (estimated average intensity of 9 METs, 4.5 MET hours per training session), followed by PRT (estimated average intensity of 5.5 METs, 4 MET hours per training session) and 15–30 minutes of interval training on stationary bicycles with peak loads of 85–95% of each participants maximum heart rate (estimated average intensity of 15 METs, 3.75 MET hours per training session. Lastly, participants engage in relaxation training lasting approximately 15 minutes.
Six machines are used during PRT: leg press, chest press, latissimus (lat.) pull down, abdominal crunch, lower back and knee extension (Technogym®, Gambettola, Italy). Muscular strength is ascertained in all six resistance training machines at baseline and at the commencement of the intervention using the 1RM test [Citation20]. Participants are encouraged to lift loads corresponding to 2–3 sets of 8–12 repetitions at 70% 1RM the first week, progressing to 80% 1RM the second week. From week three loads are lifted corresponding to three sets of 5–8 repetitions at 80–90% 1 RM. Participants who develop subjective (e.g. sensations of heaviness or swelling) or objective (e.g. visible swelling, pitting edema) signs of BCRL or experience exacerbations of an existing BCRL are instructed by the staff (physical therapists and trained nurse specialists) to decrease loads or refrain from the lat. pull down and chest press exercises and are referred to hospital- or private practice lymphedema therapists for evaluation and treatment. No systematic registration of BCRL has ever been carried out.
Therefore, using a cross-sectional design, the purpose of the present study was to investigate the prevalence of BCRL in breast cancer patients who participated in B&C from 1 January 2010 to 31 December 2011. It was hypothesized that participation in this exercise intervention utilizing heavy load PRT (> 80% 1 RM) was not associated with an increased risk of BCRL.
Material and methods
Recruitment
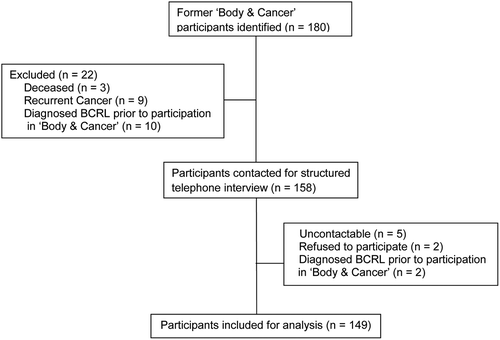
Breast cancer patients who had participated in the exercise intervention from 1 January 2010 to 31 December 2011 were identified in the B&C database (n = 180). Participants came from four university hospitals in the Copenhagen area and were eligible for the exercise intervention if they had a diagnosis of breast cancer, had received at least one cycle of chemotherapy for advanced disease or as adjuvant treatment, had a WHO performance status of 0 or 1 and otherwise had been approved to participate by the treating oncologist. Medical records were searched for a clinician diagnosis of BCRL, recurrent cancer, and mortality status. details the recruitment and exclusion process, leaving a study sample of 149 women.
Data sources
Electronic medical records. Data regarding surgery and treatment as well as BCRL, recurrent cancer and mortality status were obtained from electronic medical records.
Structured telephone interview. A structured telephone interview was administered by one of the authors (KB) a research physical therapist affiliated with the exercise intervention, and lasted on average 15 minutes. Responses were recorded on a pre-printed form. All telephone interviews were obtained within a six-week period.
The primary outcome, a clinical diagnosis of BCRL, was ascertained by asking the participant if she had been diagnosed with lymphedema. She was defined as having BCRL if she answered “yes”. If the participant reported having been diagnosed with BCRL she also was asked to report when and by whom the diagnosis was made as well as which region was affected (hand, arm, breast, torso). Demographic, treatment, and training/physical activity characteristics were also obtained. More specifically, demographic characteristics included age, current BMI, relationship status, age of children living at home, education and current occupation. Treatment characteristics included whether surgery had been performed on the dominant/non-dominant side and whether they had been introduced to post-operative exercises for breast cancer patients. Furthermore, the interview supplemented any information lacking from the medical records. Behavioral characteristics included whether the participant had performed post-operative exercises before participating in the intervention, whether they had engaged in PRT 1–3×/week between surgery and B&C, and whether they had engaged in PRT 1–3×/week post-intervention, and if so for how long, and with which loads. In addition, leisure time physical activity was explored using a validated method [Citation21].
Arm circumference measurements. For participants answering “yes” to having been diagnosed with BCRL, circumference measurements (measured at the time of lymphedema assessment) were obtained from medical records. If no circumference measurements were noted in the medical records, measurements were obtained by contacting the clinician (e.g. rheumatologist, general practitioner, lymphedema therapist, etc.) that had diagnosed the participant. No standardized protocol for measuring was used as each clinician had their own protocol ranging from five to seven measuring points. A participant was considered to have BCRL if an interlimb difference of ≥ 2 cm at to two or more measures was reported [Citation16].
B&C database. Baseline BMI and pre-illness physical activity levels [Citation21] were obtained from the database, as well as baseline and post-intervention muscular strength (1 RM) of the upper (chest press) and lower body (leg press) and adherence to the intervention.
Statistical analysis
Statistical procedures were performed using the Statistical Package for Social Sciences (SPSS) software (version 19) for Windows. Descriptive statistics are presented as proportions for categorical variables and as means and standard error (SE) for continuous variables unless otherwise noted. Mean changes in muscular strength (1 RM) after six weeks of training were assessed using a paired t-test, and were analyzed on a per-protocol (PP) basis, including only participants with baseline and six-week measurements as well as on an intention to treat (ITT) basis using baseline observation carried forward (BOCF). Point prevalence was calculated at the time of the present study [on average 14 months post-intervention (range 4–26)], and estimated retrospectively at the commencement of B&C and at 1, 2, 3, and 4 months post-B&C participation.
To compare differences between participants that had been diagnosed with BCRL and those that had not χ2-test and Fisher's exact test were used to compare categorical variables. Where relevant categorical variables were dichotomized. Continuous variables were compared using two-sample t-tests. Levene's test for equality of variances was performed and results presented use pooled variances unless otherwise noted. A two-tailed p < 0.05 was taken as evidence of statistical significance. From the literature it was known that comparison studies were carried out on AND populations alone, and therefore a sub-analysis of participants with AND was performed.
Ethical considerations
The study was performed in accordance with the Helsinki Declaration, and approved by the Danish Data Protective Agency.
Results
Participant characteristics
Of the 158 women contacted, 94.3% (n = 149) were included in the study. The mean age was 47.7 years and 14.9% had children < 7 years of age living at home. Most were in a relationship (71.8%), had higher than a secondary education (86.0%) and were currently employed (73.8%), with over half (63.6%) describing their employment as being “not physically demanding”. The mean self-reported BMI was 24.1, with 35.6% classified as overweight (> 25). 41.6% had undergone breast ablation surgery and 60.4% had received AND, with 53.7% having undergone surgery on the non-dominant side. All had undergone chemotherapy, with 94.6% having received adjuvant chemotherapy. The majority of the women had received radiotherapy (80.5%), and 79.9% had received/were receiving endocrine treatment, while 15.4% were receiving/had received trastuzumubab.
B&C participation
On average participants had initiated B&C 16.5 (range 5.6–27.6) weeks after surgery, and had undergone 3.8 (range 1–8) cycles of chemotherapy. Over half (60.4%) had an adherence rate of at least 70% (17 of 24 training days) to the exercise intervention. Both the per-protocol () and ITT (not shown) analyses revealed increases in upper and lower body muscular strength after six weeks of training. Mean time from B&C termination to telephone interview participation was 14 months, ranging between 4 and 26 months. In total 17.4% (n = 26) had been finished with the intervention up to six months, 31.5% (n = 47) between 7 and 12 months and 38.3% (n = 57) between 13 and 24 months. 12.8% (n = 19) had participated in B&C more than two years previously.
Table I. Strength outcomes after six weeks in ‘Body and Cancer’.
Self-reported leisure time physical activity levels
Over half of the participants (70.9%) reported that they had been physically active at least three hours per week, of which 7.8% had been physically active more than four hours per week pre-illness. At follow-up, on average 14 months post-intervention, a shift was seen towards more physical activity as 78.5% currently reported being physically active at least three hours per week, of which 31.5% currently were physically active more than four hours per week (p < 0.001).
Point prevalence of BCRL
The total prevalence 4–26 months post-intervention (mean 14) was 27.5% (). A sub-analysis of the AND population revealed a prevalence of 44.4%. Six percent reported that they had been diagnosed with BCRL during the intervention increasing to 17.4% at four months post-intervention. In the AND population 10.0%, and 27.8%, respectively reported a BCRL diagnosis (). All BCRL cases had received AND, with the exception of one participant. Notably, among women with a diagnosis of BCRL one reported swelling in the hand only, three in the breast only, and one in the torso only.
Table II. Point prevalence of self-reported diagnosed lymphedema and arm circumference measurements in relation to participation in the intervention. Values are numbers of participants (percentages).
Arm circumference measurements
Arm circumference measurements were taken at the time of lymphedema assessment by various clinicians at eight different facilities (two hospitals, six private practice lymphedema therapists) with varying protocols. Measurements were obtained for 38 of the 41 (92.7%) participants diagnosed with BCRL. Of these, 47.4% had an interlimb difference of ≥ 2 cm at two or more measures. Thus according to this diagnostic method, and with the criteria applied, prevalence at the study point was 12.3%, increasing from 3.4% during the intervention, to 10.3% four months post-intervention. In the AND population, point prevalence at the study period was 19.5%, ranging between 5.8% and 16.1%, respectively ().
Variable differences
A larger percentage of participants diagnosed with BCRL had a BMI > 25 (p = 0.023), had undergone AND (p = 0.000) and had received radiotherapy (p = 0.005) (). In contrast fewer had received trastuzumubab (p = 0.040). Over 90% of the participants diagnosed with BCRL had performed post-surgery exercises focusing on range of motion, at least 3×/week before initiating the B&C intervention, compared to 68% in the no BCRL group. No between group differences were noted in regard to PRT before or after B&C (), nor to strength development after six weeks of training ().
Table III. Characteristics of participants with and without BCRL (n = 149). Values are numbers (percentages) unless stated otherwise.
Variable differences in the AND population
Sub-analysis of the AND population revealed that more participants diagnosed with BCRL currently were overweight (p = 0.001), or had been overweight at baseline (p = 0.017) (). No between group differences were found in regard to post-surgery exercises. However, 83.5% of the AND population had performed these exercises in comparison to 60.3% that had received SNB (p = 0.003), thus the between group difference (No BCRL/BCRL) found in the total population was associated with axillary surgery. Similarly, no between group difference was found in regard to radiotherapy. However, 93.3% in the AND population had received radiotherapy in comparison to 62.3% in the SNB population (p = 0.000).
Table IV. Sub-analysis in the AND population (n = 90). Characteristics of participants with and without BCRL. Values are numbers (percentages) unless stated otherwise.
In contrast to the total population, no between group difference was found in regard to trastuzumubab treatment, however no association to axillary surgery was found. No between group differences were noted in regard to PRT before or after the intervention (), nor to strength development after six weeks of training ().
Discussion
The prevalence of BCRL, 4–26 months after participation in B&C, was 27.5%. Sub-analysis revealed a prevalence rate of 44.4% amongst participants who had undergone AND. More participants in the group diagnosed with BCRL were overweight and had undergone radiotherapy and AND. No associations were found between performing heavy resistance training and the development of BCRL.
Comparison to intervention studies during adjuvant treatment
Total population. The estimated prevalence of women reporting that they had been diagnosed with BCRL during the exercise intervention was 6.0%. These results are similar to Kilbreath et al. [Citation16] that reported a BCRL incidence of 7–11% (depending on the measurement method applied) after an eight-week training intervention ultilizing moderate loads. The estimated prevalence had increased to 17.4% four months post-B&C in comparison to 7–8% six months post-intervention in Kilbreath's study. However, using the same measurement method and criteria (arm circumference differences ≥ 2 cm at two or more measures) the rate of BCRL was 10.3% in the present study and 7% in the Kilbreath study.
AND population. Among participants with AND, the post-intervention prevalence was 10.0%, increasing to 27.8% four months post-B&C. Comparably, in the Kilbreath study, between 20% and 33% had developed BCRL post-intervention and 15–30% six months post-intervention. Courneya et al. reported an incidence rate of 3.7%, using water displacement, after a median of 17 weeks of PRT during adjuvant chemotherapy [Citation9]. Notably, none of these women had received radiotherapy, and no follow-up measures were reported. In a study by Sagen et al. participants initiated the intervention within the first week of surgery [Citation17]. After three months of strength training with light to moderate loads, 5% had developed BCRL (water displacement). At two years post-surgery the incidence rate was 13%. This stands in contrast to the present study with 44.4% reporting that they had been diagnosed with BCRL 8–28 months post-surgery. However due to inherent differences in study design (RCT/cross-sectional), differing measurement methods and treatment burden, caution should be applied when interpreting these diverging results.
RCT versus cross-sectional studies. Inherent differences in study design make it difficult to compare results from the controlled framework of a RCT with results from a cross-sectional study. Participants were excluded from the RCTs if they had undergone reconstructive surgery [Citation9], had metastatic cancer [Citation16,Citation17], or bilateral breast cancer [Citation16], or if they had a pre-existing upper limb impairment [Citation16,Citation17]. Furthermore, participants were screened and excluded if they presented with BCRL. In the present study, none of these exclusion criteria were applied and no pre-intervention BCRL screening took place, thus participants could have initiated B&C with an undiagnosed BCRL. Moreover, BCRL was in focus in the RCTs, and participants received treatment upon any signs or symptoms of BCRL during both the intervention and follow-up periods. This likely decreased the incidence of BCRL in comparison to the present study where participants were encouraged to seek treatment by the B&C staff upon symptoms of BCRL, but “were on their own” after the intervention period.
Measurement methods. It is well established that the diagnostic method used in a given observational or intervention study influences the incidence or prevalence found [Citation3]. This is illustrated in a recent study that found baseline prevalence rates ranging from 22% (arm circumference) to 52% (self-reported swelling) depending on the four measurement methods applied [Citation3]. Similarly, Ahmed et al. reported baseline BCRL prevalence rates of 17.4% (arm circumference), 43.4% (self-reported swelling), and 30.4% (self-reported clinician diagnosis) [Citation22]. In the present study, under half (47.4%) of the women that had reported a clinician diagnosis of BCRL presented with an interlimb difference ≥ 2 cm at two or more measures, indicating that if this measurement method had been used as the primary measure, prevalence rates had been considerably lower.
Treatment burden. Breast cancer treatment is tailored to the individual breast cancer status, thus women treated for breast cancer is a heterogeneous group [Citation4]. This is exemplified in a Danish population-based study that found that 1–3 years post-surgery, BCRL prevalence rates ranged from 13% to 65% based on treatment burden alone [Citation4]. The study illustrates well the complexity regarding overall prevalences in this group, and should be taken into consideration in the interpretation of prevalence and incidence rates.
Known risk factors
In accordance with risk factors identified by Disipio et al. as being supported by a high level of evidence [Citation1], more women in the group diagnosed with BCRL had a current BMI > 25, and had received AND. Moreover, sub-analysis of the AND population revealed that more women had a BMI > 25 at baseline and were currently overweight in the group diagnosed with BCRL. In the total population, more women that had received radiotherapy reported a diagnosis of BCRL. This is also in accordance with Disipio et al. that found a moderate level of evidence supporting radiotherapy as a risk factor [Citation1]. However, this association was not observed in the AND population, perhaps due to a small sample size. Nonetheless, these findings are in accordance with results found by Sagen et al. [Citation17], who found that being overweight was the only predictor of developing BCRL among study participants who all had received AND.
An unexpected finding, the results showed that more women had received trastuzumubab in the no BCRL group. This difference was not observed in the AND population, however no association to axillary surgery was found. No studies were identified by the author that have observed a protective effect of this adjuvant treatment, and as this study is merely a descriptive study with a small population no conclusions can or should be drawn. Rather it should be used as an observation and perhaps a catalyst for further study.
Physical activity levels
Questions regarding leisure time physical activity levels were validated [Citation21] and had been administered to participants at the initiation of the B&C training intervention, and could therefore be used to describe any trends in time.
In total 63.1% reported pre-illness physical activity levels corresponding to at least three hours per week, and 7.8% over four hours per week. Physical activity levels at follow-up (4–26 months post- intervention) had shifted to an increase in physical activity with 47.0% reporting that they presently were exercising at least three hours per week, and 31.5% exercising over four.
However, these results are likely inflated due to pleasing bias as approximately half of the participants had previously met the author due to her affiliation with B&C as a trainer. Nonetheless, this is an interesting finding as previous studies have found that women who undergo treatment for breast cancer tend to decrease physical activity levels [Citation23]. More importantly this shift was also seen in participants diagnosed with BCRL, indicating that maintaining and possibly increasing a physically active lifestyle is possible with BCRL. Furthermore, this finding indicates that participation in an intensive, multimodal, six-week intervention promoting physical activity during adjuvant treatment perhaps can play a role in lasting lifestyle changes.
Post-surgery exercises
Self-reported adherence to post-surgery exercise was high, with 75.2% of the total population and 83.5% of the AND population performing these exercises at least three times per week before initiating B&C. Despite this, 90.2% of the participants that developed BCRL reported that they regularly had performed these exercises, thus no protective effect was indicated. However, post-operative exercises focusing on mobility of the shoulder joint and stretching of muscles related to breast cancer surgery play an important role in restoring normal function of the affected limb [Citation24] and should therefore be an integrated part of a rehabilitation program.
PRT during and after B&C
Strength outcome measures and adherence related to B&C. No between group differences in post-intervention 1 RM strength or adherence were found. These results indicate that there was no difference in strength training intensity between the women that did and did not develop BCRL.
PRT after B&C. Over 50% reported that they had continued to perform PRT 1–3×/week for a minimum of three months, with no between group differences. These results are in line with previous intervention studies, finding no associations between PRT and an increased risk of BCRL. Approximately 30% reported that they had lifted loads of 5–8 repetitions (heavy), with no between group differences. This finding indicates that heavy PRT after B&C was not associated with a self-reported clinician diagnosis of BCRL, however further investigation using a robust design is warranted.
Strengths and limitations
The participants. A total of 83% of the identified B&C participants made up the study population. Twelve women with pre-existing BCRL were excluded as one of the main objectives of the study was to describe association between participation in B&C and the development of BCRL. Thus, when considering the prevalence of BCRL in the study population, these participants should be taken into account.
Moreover, due to ethical considerations, nine women were not contacted as it had been noted in the medical records that a recurrent cancer had recently been detected or was under investigation, and three were deceased. A further two declined to participate for personal reasons, and five were uncontactable. Thus, the BCRL status of these women is not known.
Design. As this study utilized a cross-sectional design, and with a small study sample, it does not offer the possibility of drawing conclusions regarding causality, but can merely describe factors associated with identified cases of BCRL as defined in this study. Thus, though results do not indicate an association between heavy resistance training and the development of BCRL, conclusions regarding the “safety” of heavy resistance training cannot be drawn. Nonetheless, the observations from this study are in line with studies from Cormie et al. [Citation18,Citation19], who found that heavy load PRT was a safe training modality for persons with an existing arm lymphedema, lending credibility to the results of the present study.
Structured telephone interview. The response rate to the telephone interview was high (94.3%). Furthermore, there was a 100% completion rate amongst responders indicating that this was an evident method for obtaining data. The structured telephone interview was designed for the present study and was not validated. Furthermore, some of the questions were retrospective in nature, thus a risk of recall bias exists.
BCRL definition considerations. Ideally, objective measurements would have been performed on all participants, however, this was beyond the scope of the study and was not possible. Thus, reporting a clinical diagnosis of BCRL was utilized as the primary definition, despite numerous uncertainties. Firstly, this provides just one estimate of prevalence and therefore does not give the possibility to determine whether it was transient lymphedema dissipating over time, or chronic BCRL. A longitudinal study (n = 211) evaluating BCRL on five occasions up to 18 months post-surgery, found that almost 60% of the women that had showed evidence of BCRL, had transitory symptoms whereby the lymphedema dissipated [Citation2]. This is important to bear in mind when comparing the follow-up incidence estimates in the Kilbreath and Sagen studies where all participants were evaluated anew, whereby transient lymphedema cases were identified and therefore not included as an incident BCRL case. In the present study all BCRL cases were accumulated and thus, the final estimated prevalence is likely overestimated. Second, BCRL was determined by at least eight different clinicians, with no standardized protocols, cut-offs or criteria for when a participant presented with BCRL, lending uncertainty as to the real prevalence in the study population. However, in comparison to studies with well-defined cut-offs and controlled frameworks, the present study is pragmatic and reflects the reality of clinical practice. Finally, in the present study BCRL was not limited to the arm, and should be considered when interpreting the results of the study.
Despite the limitations, the present study with its high response rate and 100% completion rate amongst responders, offers conceivable prevalence estimates of BCRL amongst former participants of this exercise intervention. Furthermore, the data obtained are consistent, with no contradictory findings, lending credibility to the results.
Studies investigating BCRL are confronted with a number of challenges. Variations in treatment burden alone, influence prevalence rates. Moreover utilization of different measurement methods and criteria applied to define chronic BCRL, thwart comparisons between studies and limits the knowledge gained, thus international consensus needs to be found. Nonetheless, irrespective of the diagnostic criteria applied, studies have found PRT to be safe following breast cancer, with this study adding to the growing database. There is considerable rational for promoting PRT during adjuvant treatment as PRT performed alone or in combination with other exercise modalities has been shown to relieve a number of chemotherapy-induced side effects [Citation9,Citation16,Citation20]. Furthermore, though strength gains are seen with lighter loads, heavy PRT is an effective training method and necessary for achieving strength gains in trained individuals [Citation25].
Conclusion
The prevalence of self-reported clinician diagnosed BCRL, 4–26 months after participation in a rehabilitation exercise intervention utilizing heavy resistance training, was 27.5%. Sub-analysis revealed a prevalence rate of 44.4% amongst participants who had undergone axillary node dissection.
There appears to be no association between performing heavy resistance training during adjuvant therapy, and the development of BCRL. However randomized controlled trials should be performed to confirm this observation.
Acknowledgments
Thanks to all the participants who donated their time and shared their experiences.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol 2013; 14:500–15.
- Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J Clin Oncol 2008;26:3536–42.
- Hayes SC, Speck RM, Reimet E, Stark A, Schmitz KH. Does the effect of weight lifting on lymphedema following breast cancer differ by diagnostic method: Results from a randomized controlled trial. Breast Cancer Res Treat 2011; 130:227–34.
- Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment – a nationwide study of prevalence and associated factors. Breast 2010; 19:506–15.
- Kwan ML, Cohn JC, Armer JM, Stewart BR, Cormier JN. Exercise in patients with lymphedema: A systematic review of the contemporary literature. J Cancer Surviv 2011;5: 320–36.
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exer 2010;42:1409–26.
- Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, et al. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat 2010;121:421–30.
- McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: A pilot study. J Clin Oncol 2003;21:463–6.
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–404.
- Battaglini C, Bottaro M, Dennehy C, Rae L, Shields E, Kirk D, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J 2007;125:22–8.
- Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: A randomized, controlled trial. Breast Cancer Res Treat 2011;127:447–56.
- Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. New Engl J Med 2009;361: 664–73.
- Hayes SC, Reul-Hirche H, Turner J. Exercise and secondary lymphedema: Safety, potential benefits, and research issues. Med Sci Sport Exer 2009;41:483–9.
- Baechle TR, Earle RW, Wathen D. Essentials of strength training and conditioning. In: Baechle TR, Earle RW, editors. Human Kinetics, 2nd ed. Champaign IL: National Strength and Conditioning Association; 2000.
- Anderson RT, Kimmick GG, McCoy TP, Hopkins J, Levine E, Miller G, et al. A randomized trial of exercise on well-being and function following breast cancer surgery: The RESTORE trial. J Cancer Surviv 2012;6: 172–81.
- Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: A randomized controlled trial. Breast Cancer Res Treat 2012;133:667–76.
- Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol 2009;48: 1102–10.
- Cormie P, Galvao DA, Spry N, Newton RU. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr Cancer Ther 2013;12:423–32.
- Cormie P, Pumpa K, Galvao DA, Turner E, Spry N, Saunders C, et al. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: A randomised controlled trial. J Cancer Surviv 2013;7:413–24.
- Adamsen L, Quist M, Andersen C, Moller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. Br Med J 2009; 339:b3410.
- Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation 1968;38:1104–15.
- Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol 2006;24:2765–72.
- Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J Clin Oncol 2008;26:3958–64.
- McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev 2010:CD005211.
- American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sport Exer 2009;41:687–708.