To the Editor,
Despite recent progress in the knowledge in cancer biology and considerable expenditure and efforts in the development of new ‘targeted’ cancer drugs, progress has been limited in the major cancer types; the newly introduced drugs mostly provide very modest benefit at high costs, both in terms of healthcare expenditure and patient adverse effects [Citation1]. Drug repositioning, i.e. the finding of new indications for drugs in development or use in other diseases is an attractive approach to more efficient drug development by making use of the preclinical and clinical knowledge already available, allowing for considerable shortcuts in many steps in the drug development process [Citation2].
In an ongoing drug screening project in our laboratory aiming at repositioning drugs for use in advanced colorectal cancer, we observed high activity in colon cancer models in vitro for several members of the bendazole group of compounds, including the approved antihelmintic drug mebendazole (tradename Vermox).
Shortly following this observation, a 74-year-old man with metastatic colon cancer was considered for further treatment at Department of Oncology, Uppsala University Hospital. He had been healthy until emergency surgery after presentation with ileus in 2011. An advanced sigmoid colon cancer was radically resected. Histopathological examination showed a moderately differentiated adenocarcinoma with KRAS mutation. Synchronous multiple bilateral lung and paraaortic lymph node metastases were observed and the patient started palliative chemotherapy with a combination of capecitabine, oxaliplatin and bevacizumab. A partial remission was observed at CT evaluations after two and four months of treatment. Following a period of maintenance treatment with capecitabine alone, progressive disease was observed in August 2012. Capecitabine, oxaliplatin and bevacizumab was restarted and resulted in stable disease at the first evaluation. However, this treatment was stopped due to intolerable oxaliplatin-induced neuropathy. Second line treatment with capecitabine and irinotecan was started in December 2012. At first evaluation March 2013, significant progression was observed in the lungs and abdominal lymph nodes and there were new partly poorly defined liver metastases up to 8 cm in diameter (). Despite the progress the patient was still in excellent performance status and had essentially no disease-related symptoms, except for the therapy-induced neuropathy.
Figure 1. Axial computerized tomography at baseline showing multiple liver (A) and lung (C) metastases (arrows) and good regression at first evaluation (B and D), following 6 weeks of treatment with mebendazole.
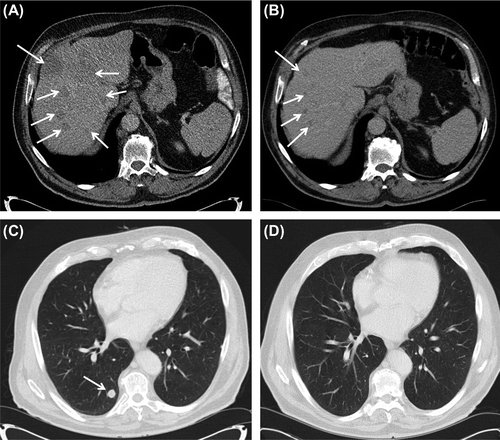
In this situation with no standard therapy left, the drug screen observation in mind, the recent preclinical finding of antitumour effects of mebendazole in a mice model [Citation3] and a case report indicating anti-tumour effect from mebendazole in a patient with metastatic adrenocortical carcinoma [Citation4], the patient, after informed consent, started mebendazole at the standard antihelmintic dose of 100 mg twice daily, to be continued for six weeks. The patient experienced no adverse effects from the treatment and at CT evaluation May 2013 there was near complete remission of the metastases in the lungs and lymph nodes and a good partial remission in the liver (). At this stage, the liver enzymes AST and ALT were found elevated up to five and seven times above upper limit of normal and mebendazole was temporarily stopped and then reintroduced at half dose. Liver enzymes slowly decreased and the patient still reported no adverse effects from mebendazole. The disease was stable at a new CT, confirming the response observed earlier. The patient is now on drug holiday, pending a new CT.
We conclude that mebendazole is seemingly a promising ‘new’ drug for treatment of advanced colorectal cancer. A number of pharmacodynamic, clinical trial, drug formulation and dosing issues need to be addressed before mebendazole might become an established drug in this indication. However, the case, together with its preclinical background, illustrates the potential of drug repositioning to improve the development of new and better cancer therapy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stewart DJ, Kurzrock R. Cancer: The road to Amiens. J Clin Oncol 2009;27:328–33.
- Ashburn TT, Thor KB. Drug repositioning: Identifying and developing new uses for existing drugs. Nature Rev Drug Disc 2004;3:673–83.
- Mukhopadhyay T, Sasaki J, Ramesh R, Roth JA. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin Cancer Res 2002;8:2963–9.
- Dobrosotskaya IY, Hammer GD, Schteingart DE, Maturen KE, Worden FP. Mebendazole montherapy and long-term disease control in metastatic adrenocortical carcinoma. Endocrine Practice 2011;17:e59–62.
