To the Editor,
Gastric cancer is known for its aggressive natural history and most patients present with advanced, inoperable or metastatic disease. In the absence of curative treatment modalities, systemic chemotherapy can be considered as a reasonable treatment option for these patients. Previous studies showed that, in selected patients, chemotherapy is superior to ‘best supportive care’ in terms of prolonged survival, reduction of disease-related symptoms and improved quality of life [Citation1]. Therefore, national and international guidelines recommend palliative chemotherapy with a two or three drug regimen for patients with metastatic gastric cancer [Citation2,Citation3].
In patients with metastatic gastric cancer, dissemination to the peritoneum is one of the most common sites. Up to 39% of the gastric cancer patients presents with metastatic disease and 14% of all the newly diagnosed patients presents with peritoneal carcinomatosis (PC) [Citation4]. The efficacy of palliative chemotherapy for the subset of patients with PC from gastric origin is not known. It is hypothesized that the effect of intravenous chemotherapy on peritoneal metastases is limited due to the peritoneal blood barrier, and peritoneal dissemination is established as an adverse prognostic factor [Citation5]. Comprehensive data regarding the use and effectiveness of systemic chemotherapy for this subset of patients in particular is virtually absent. Therefore, the aim of this population-based study was to evaluate trends in systemic treatment and survival of patients with PC of gastric origin.
Material and methods
The Eindhoven Cancer Registry registers all newly diagnosed cancer patients in the southern part of the Netherlands, including 10 community hospitals, six pathology departments and two radiotherapy institutions, comprising 2.4 million inhabitants. All patients diagnosed between 1995 and 2011 with an adenocarcinoma of gastric origin were included. These 5220 patients were previously described [Citation4]. Information on patient and tumor characteristics is routinely extracted from the medical records by specially trained administrators of the cancer registry. Anatomical sites of distant metastases are registered according to the International Classification of Disease-Oncology (ICD-O). By means of an independent case ascertainment method, the completeness of the registration is estimated to exceed 95% [Citation6]. Chemotherapy (yes vs. no) was defined as receiving intravenous cytostatic drugs of any kind. The vital status of all patients was assessed on January 1, 2012 through merging with the Municipal Administrative Databases in which all deceased and emigrated persons in the Netherlands are registered.
Statistical analysis
Differences between patients who were treated with palliative chemotherapy and patients who were not, were tested by means of a χ2-test. To investigate trends in treatment and survival, patients were categorized into four groups by period of diagnosis: 1995–1998, 1999–2002, 2003–2006 and 2007–2011. Survival time was defined as the time from cancer diagnosis until death; patients still alive at January 1, 2012 were censored. Crude survival was determined by the Kaplan-Meier method and compared using a log-rank test. Cox regression analysis, adjusting for age, gender, period of diagnosis, co-morbidity, tumor differentiation grade, tumor stage, lymph node stage, and surgery, was used to determine the relationship between chemotherapy and two-year mortality among patients with PC. A hazard ratio (HR) was provided with the 95% confidence interval (CI). All tests of statistical significance were two sided. SAS/STAT statistical software (SAS system 9.3, SAS Institute, Cary, NC, USA) was used for all analyses.
Results
Between 1995 and 2012, 5220 patients were diagnosed with gastric cancer, of whom 2029 patients (39%) had metastatic disease at presentation [Citation4]. Of these patients 706 patients (34%) were diagnosed with PC, of whom 491 patients had PC as the only metastatic site and 215 patients had PC combined with other metastases. In total 168 patients (24%) were treated with palliative chemotherapy. Younger patients, patients with less co-morbidities and patients with lower N-stage were significantly more likely to be treated with chemotherapy (Supplementary material Table I, available online version at http://informahealthcare.com/doi/abs/10.3109/ 0284186X.2013.850740). Furthermore, the percentage of patients treated with chemotherapy increased over time (p < 0.001, ). In the period 1995–1998, 11% of the patients with PC were treated with chemotherapy as compared with 42% in the most recent period.
Figure 1. Percentage of patients with gastric cancer treated with chemotherapy, according to period of diagnosis. PC only: PC without other metastases; PC other: PC and other metastases.
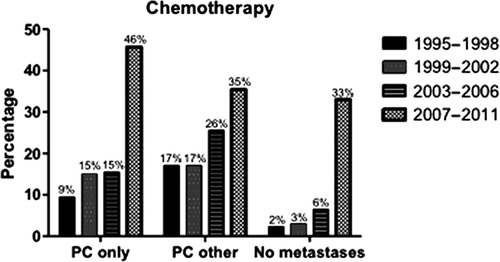
Table I. Median survival of patients with PC treated with chemotherapy and without chemotherapy.
Median survival of patients with PC was 4 months. For those who received chemotherapy it was 7.7 months compared to 3.4 months in patients who were not treated (p < 0.001, ). Crude median survival did not significantly increase over time among all PC patients and among patients treated with chemotherapy (p = 0.740 and p = 0.310, respectively). Crude survival of patients who did not receive chemotherapy decreased over time (p < 0.0001). After adjusting for age, gender, period of diagnosis, co-morbidity, tumor differentiation grade, tumor stage, lymph node stage, and surgery, patients with PC who were treated with palliative chemotherapy had a reduced risk to die within two years after gastric cancer diagnosis (HR = 0.48, 95% CI 0.38–0.60, ).
Table II. Multivariable logistic regression modeling the risk to die within 2 years after gastric cancer diagnosis among patients with PC of gastric origin.
Discussion
Gastric cancer is the second most common cause of death from cancer worldwide, accounting for 740 000 deaths per year [Citation7]. All over the world, epidemiological features of gastric cancer patients are changing. In the Netherlands the age-standardized incidence rates decreased in the last decades but stage distribution worsened over time [Citation8]. As a result, a growing proportion of patients now presents with locally advanced or metastatic disease with peritoneal dissemination being the most commonly affected metastatic site. The growing experience with combination chemotherapy in the treatment of gastric cancer, especially after the publication of the REAL-2 and MAGIC-trial in 2005 and 2006, has resulted in a strong increase in the administration of chemotherapy for advanced gastric cancer in the Netherlands since 2007 [Citation9,Citation10]. Also in the current study, the usage of chemotherapy appeared to have significantly increased over time. However, crude median survival did not significantly increase over time among PC patients. This observation suggests that palliative chemotherapy is of limited value for gastric cancer patients suffering from PC. This seems in conflict with the multivariable logistic regression model showing that treatment with chemotherapy results in a reduced risk to die within two years after the diagnosis. However, additional patient characteristics such as performance score, nutritional status and disease-related symptoms also influence the prescription of palliative chemotherapy and therefore it is conceivable that selection bias probably has played a major role.
Previously, it has been hypothesized that the effect of intravenous chemotherapy may be limited in PC patients due to the peritoneal blood barrier. Ross et al. [Citation11] revealed that patients with PC had a 15% response rate to chemotherapy compared to a response rate of 43% in patients with other metastatic sites of advanced gastric cancer. Our study demonstrated that younger patients, patients with less co-morbidities and patients with a lower N-stage were more likely to be treated with chemotherapy, which is in line with previous studies, also revealing reluctance to prescribe chemotherapy to old and frail patients [Citation12].
Only few clinical studies have reported on the effect of chemotherapy in gastric cancer patients with PC. In a study reporting on 172 patients with PC of gastric origin receiving chemotherapy, an improved one-year survival of 23.9% versus 4.6% was reported, but this survival benefit was not significant in the multivariate analysis (p = 0.082) [Citation13]. Shigeyasu et al. [Citation14] established a median survival of 15.3 months in a small phase II study, including 19 patients with PC of gastric origin treated with S-1 and docetaxel. Izuishi et al. [Citation15] reported retrospectively collected data of a palliative gastrectomy and chemotherapy and found that S-1 containing chemotherapy showed a significant survival benefit over 5-FU containing chemotherapy. However, the most promising results were reported by studies using multi-modality treatment including cytoreductive surgery and intra-peritoneal administration of chemotherapy, showing that median survival improved from 7.9 to 15 months for patients with completeness of cytoreduction [Citation16].
In conclusion, the usage of chemotherapy increased in patients with PC of gastric origin but this did not result in prolongation of survival on a population-based level. Therefore, the beneficial effect of current chemotherapy regimens remains questionable at least in this patient category. Given the bad prognosis of these patients if left untreated, further research should be performed to optimize therapy, which may include multi-modality treatment with intraperitoneal chemotherapy.
Supplementary material Table I
Download PDF (100.9 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wagner, AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010; CD004064.
- National Comprehensive Cancer Network, NCCN Guideline: Gastric Cancer 2012.
- Landelijke werkgroep Gastro-Intestinale Tumoren, Dutch Guideline: Gastric Carcinoma 2009.
- Thomassen I, van Gestel YRBM, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, risk-factors and prognosis. Int J Cancer Epub 2013 Jul 5.
- Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, et al. Prognostic model to predict survival following first- line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol 2007;18:886–91.
- Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol 1993;22:369–76.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354–62.
- Dassen AE, Lemmens VE, van de Poll-Franse LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, et al. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: A population-based study in the Netherlands. Eur J Cancer 2010;46:1101–10.
- Chong G, Cunningham D. Can cisplatin and infused 5-fluorouracil be replaced by oxaliplatin and capecitabine in the treatment of advanced oesophagogastric cancer? The REAL 2 trial. Clin Oncol 2005;17:79–80.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20.
- Ross P, Nicolson M, Cunningham D, Ishibashi H, Mizumoto A, Miura M, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002;20:1996–2004.
- van Gils CW, Koopman M, Mol L, Uyl-de Groot CA, Punt CJ. Adjuvant chemotherapy in stage III colon cancer: Guideline implementation, patterns of use and outcomes in daily practice in The Netherlands. Acta Oncol 2012;51: 57–64.
- Zhu G, Zhang M, Zhang H, Gao H, Xue Y. Survival predictors of patients with gastric cancer with peritoneal metastasis. Hepatogastroenterology 2010;57:997–1000.
- Shigeyasu K, Kagawa S, Uno F, Nishizaki M, Kishimoto H, Gochi A, et al. Multicenter phase II study of S-1 and docetaxel combination chemotherapy for advanced or recurrent gastric cancer patients with peritoneal dissemination. Cancer Chemother Pharmacol 2013;71:937–43.
- Izuishi K, Haba R, Kushida Y, Kadota K, Takebayashi R, Sano T, et al. S-1 and the treatment of gastric cancer with peritoneal dissemination. Exp Ther Med 2011;2: 985–90.
- Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J Surg Oncol 2011;104:692–8.
