Abstract
Background. Recommended treatment for lymphoblastic lymphomas, a highly aggressive, relatively rare lymphoma entity predominantly seen in teenagers and young adults, includes acute lymphoblastic leukemia (ALL)-like induction chemotherapy. Whether these patients should be consolidated with maintenance chemotherapy or autologous stem cell transplantation (Auto-SCT) and the use of radiotherapy are matters of debate. Methods. We reviewed treatment and outcome for 25 consecutive patients above the age of 15 years with lymphoblastic lymphoma (T-lineage; T-LBL, n = 19; B-lineage; B-LBL, n = 6) seen at a single center during a 12-year period (1999–2011). Patients were given an ALL-like chemotherapy induction regimen, and responding patients were consolidated with Auto-SCT and local radiotherapy when applicable. Results. Median age at diagnosis was 33 years (range 15–65). Seventeen of the T-LBL patients had a mediastinal mass, three patients had central nervous system (CNS) involvement. Chemotherapy with intensified CNS prophylaxis induced an overall response rate of 92% (CR 84%, PR 8%). In total 23/25 (92%) patients underwent Auto-SCT in first remission while 13 of 14 eligible patients with mediastinal involvement received local radiotherapy. Twenty percent of the patients had hepatotoxicity grade 3–4 and 32% thromboembolic events (TE). Two patients (8%) died of treatment-related toxicity. One patient had progressive disease and died of lymphoma. Three patients have relapsed, but two of these (both B-LBL) are currently alive in second CR after Allo-SCT. With a median follow-up of 98 months (range 1–163) the 5- and 8-year PFS and OS are 76% and 84%, respectively. Conclusions. Combined intensive ALL-like induction and early consolidation chemotherapy followed by Auto-SCT and local radiation therapy resulted in high sustained cure rates.
Adult lymphoblastic lymphoma (LBL) is considered to be a rare disease entity, constituting only 2% of all non-Hodgkin's lymphomas (NHL). The disease primarily affects patients in their late teens and early adulthood and is characterized by a rapid onset. Based on the extent of bone marrow (BM) involvement (< 25% infiltration of lymphoblasts), LBL can be discriminated from acute lymphoblastic leukemia (ALL). In terms of morphology, immunophenotype and genetic features, these precursor B/T cell malignancies are regarded as different entities but with similar aggressive clinical behavior [Citation1]. B-cell lineage is the more frequent in ALL, while the opposite is the case for the LBL. Thus, T-LBL constitutes 80–90% of the lymphoblastic lymphomas and is 5–10 times more common than B-LBL [Citation1,Citation2].
Treatment results were rather dismal with standard CHOP-based chemotherapy [Citation3]. Some improvements were seen when NHL induction regimens were followed by high dose therapy and Auto-BMT/SCT or maintenance chemotherapy with long-term survival rates of 32–50% [Citation4–10]. Results from more intensive ALL-like induction regimens showed even better outcomes [Citation11–13]. Most centers agree that some form of late consolidation therapy is needed in LBL, by intensified cycles of chemotherapy followed by either maintenance chemotherapy, Auto-SCT or Allo-SCT. Only one randomized phase III trial comparing consolidation by Auto-SCT with a 2½ -year maintenance chemotherapy regimen has been performed [Citation14]. Auto-SCT showed a trend for improved progression-free survival (PFS), whereas no difference in overall survival (OS) was observed between the two arms. At present there is no consensus as to the role of Auto-SCT in the treatment of LBL.
T-cell LBL often presents with a large mediastinal mass. This location is also a common site of relapse [Citation15,Citation16]. Therefore many centers recommend mediastinal irradiation (RT) following intensive chemotherapy [Citation15–17]. While only 2–7% of patients present with central nervous system (CNS) involvement, one third of patients not given CNS prophylaxis experience a CNS relapse [Citation18].
Our institution is responsible for treatment of LBL in South-East Norway, covering more than 50% of the Norwegian population. Our strategy involves the use of four months of an intensive ALL chemotherapy regimen including systemic and intrathecal CNS prophylaxis, followed by Auto-SCT and mediastinal RT. The intent of this retrospective analysis was to review the treatment results from the start of this treatment strategy during a 12-year period, (1999–2011), for the age group 15–65 years as older patients would not tolerate the treatment. Twenty-five patients have been treated accordingly at our institution during this time period.
Patients, materials and methods
Study group
Since 1980 data from all lymphoma patients treated at our hospital have been registered prospectively. Results in this study are based on information retrieved from this register, supplemented with information from patient medical records. Patients aged 15–65 with B- or T-LBL admitted during a 12-year period (1999–2011) and with less than 25% blasts in the BM were included [Citation1]. One 16-year-old patient with a T-cell LBL diagnosed in 2007 who preferred maintenance chemotherapy to Auto-SCT, is in a continuous complete remission (CR), but is not included in this retrospective analysis.
Diagnostic and staging procedure, disease response monitoring and toxicity
Patients were staged according to the Ann Arbor system [Citation19]. Standard work-up consisted of computer tomography (CT) scans of neck, thorax, abdomen and pelvis, BM biopsy and cerebrospinal fluid analysis by cell count, cytology and/or flow cytometry. Disease response was monitored after six weeks of induction therapy, before and after Auto-SCT and after RT (if given) including CT scans, BM biopsy and cerebrospinal fluid analysis if positive at diagnosis. Patients who achieved a PR or a CR at six weeks continued treatment according to the early consolidation phase of the Hammersmith regimen (), followed by Auto-SCT as a late consolidation [Citation20]. Toxicity during treatment was assessed retrospectively from medical records and graded according to the Common Terminology Criteria for Adverse Events v3.0.
Table I. The intensified Hammersmith regimen.
Treatment
Induction, early consolidation, stem cell harvest and Auto-SCT as late consolidation. Patients were treated according to a Norwegian intensified ALL regimen adapted from the MRC Leukemia Unit, Royal Postgraduate Medical School, London, UK [Citation20,Citation21]. The choice of our ALL-induction regimen was based on national consensus and favorable survival data for patients with ALL under the age of 65 years, validated in a recent retrospective analysis [Citation21]. The iHammersmith (iH) regimen replaced other, possibly less intensive ALL regimens in use for LBL before 1999. Additional modifications were made to further intensify the CNS prophylaxis from 2005: intra-thecal MTX 15 mg, cytarabine 40 mg and prednisolone 10 mg was administered seven times, and the dose of i.v. MTX at week 10 and 12, was increased to 1.5 g/m2. This regimen was named iH, for details see . In short, the induction phase (day 1–29) consisted of oral prednisolone and i.v. vincristine, L-asparaginase, doxorubicin and cyclophosphamide. For patients having CD20 + B-LBL (2/6), rituximab was added both during induction and consolidation. In one patient being t(9;22) positive, imatinib was given as maintenance therapy after Auto-SCT. The iH RAT consolidation course was used as mobilization regimen, followed by G-CSF 10 μg/kg/day, until successful PBSC harvest. A minimum of 2.0 × 106 CD34 + cells/kg body weight was required for Auto-SCT. The standard BEAM conditioning regimen with autologous stem cell support was given for patients in CR/CRu or PR [Citation22]. There was no routine use of G-CSF after Auto-SCT.
Radiotherapy
Patients diagnosed with a mediastinal mass of any size, were to receive mediastinal RT, (24.0–32.0 Gy in 2 Gy fractions, 5 fractions per week), preferably after Auto-SCT. The radiation field included the initial bulky mass with a margin of 2–3 cm cranially and caudally and with a margin from the residual mass or the mediastinum after chemotherapy axially of 1–2 cm. The radiation fields were given with a weight of the anterior: posterior field = 2:1 or, during the later time period as CT-based dose planning. Other indications for radiotherapy were involvement of testes and/or skeletal lesions. Cranial (CRT) and/or spinal axis irradiation were not routinely used, neither as prophylaxis nor in patients with CNS involvement.
Statistics
Continuous variables such as age and observation time were presented as median and range. OS was calculated from date of diagnosis to death of any cause. PFS was calculated from diagnosis to progressive disease, relapse or death of any cause, whichever occurred first. Survival was analyzed with the Kaplan-Meier method [Citation23]. Differences between groups were analyzed with the LogRank method. Statistics were performed using the SPSS 20 software package (SPSS Inc., Chicago, IL, USA).
Results
Patients
From 1999 to 2011, 25 LBL patients aged 15–65 years were registered; 15 males and 10 females. Median age at diagnosis was 33 years (range 15–65). Seventy-six percent had the T-LBL subtype (n = 19) and 24% had B-LBL (n = 6). Most T-LBL patients (89%) had a mediastinal mass. Nineteen patients (76%) had stage III–IV disease and three (12%) CNS involvement. Patient characteristics at diagnosis are summarized in .
Table II. Patient characteristics.
Induction, early consolidation and Auto-SCT as a late consolidation
The majority of patients in this study (23/25 pts) received iH for induction and consolidation (). Two patients (one T-LBL and one B-LBL), both aged 15 years and with CNS involvement were treated with other regimens including more intensive CNS treatment than iH. They had no treatment delay and proceeded to Auto-SCT in first remission. Two patients were critically ill when admitted to hospital, and received one course of alternative initial chemotherapy before iH. Most patients (19 of 23) completed induction and early consolidation therapy according to iH protocol, while two patients had a delay of more than two weeks; one had PD and one a toxicity-related death. Ninety-two percent (23/25) of the patients underwent successful stem cell harvest and proceeded to Auto-SCT. Five patients had Auto-SCT postponed for up to 12 weeks, for different reasons, and had to receive maintenance chemotherapy or RT during this period.
Table III. Therapy, observation time and outcome.
Radiotherapy
Seventeen patients had mediastinal disease, two died of treatment-related toxicity and one experienced progressive disease. Thirteen of 14 eligible patients with mediastinal involvement received radiotherapy 24.0–32.0 Gy. All 13 patients were in CR prior to radiotherapy. Three patients received testicular irradiation and three received skeletal irradiation. One of the three patients with CNS involvement received CNS axis irradiation. Both patients with bulky B-LBL received radiation.
Toxicity
All patients had hematological toxicity grade 4 and there were nine incidents of verified bacteremia during induction and early consolidation. During Auto-SCT all patients had febrile neutropenia and six had verified bacteremia. There were six cases of opportunistic infections; one pulmonary aspergillosis and two cases of hepatosplenic candidiasis prior to Auto-SCT, two incidents of CMV reactivations during Auto-SCT and one Pneumocystis jirovecii pneumonia (PCP) during radiotherapy.
Twenty percent of the patients experienced hepatotoxicity grade 3–4 during induction chemotherapy, most cases in relation to L-asparaginase therapy. Polyneuropathy was common with grade 2 in 20% and grade 3–4 in 24% of patients, respectively. Thirty-two percent experienced thromboembolic events (TE); catheter-associated thrombosis, n = 3, deep venous thrombosis or pulmonary embolism, n = 4 and sinus vein thrombosis, n = 2 (one fatal in a 65-year-old woman). One T-LBL patient aged 46 years died in multiorgan failure during aplasia after Auto-SCT.
Three male patients aged 26, 30 and 32 years developed symptomatic osteonecrosis; one of the two patients with bilateral femoral head necrosis had both hips replaced by surgery.
No secondary cancers are reported. None has yet developed heart failure or permanent clinically reduced lung function. Three females got pregnant after treatment, one by egg donation, the two others were 16 years and 24 years at the time of Auto-SCT. Four males have become fathers, either by cryopreserved sperm or fresh samples.
Efficacy results
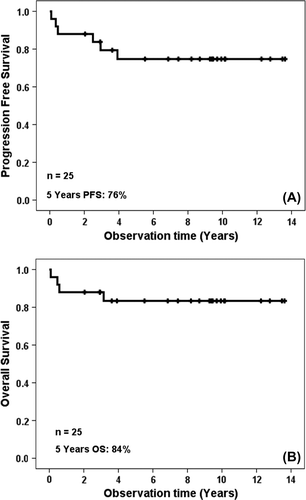
The iH regimen for induction and early consolidation resulted in an overall response rate of 92% (CR 84%, PR 8%). One patient had progressive disease and one died of toxicity (sinus vein thrombosis) during induction treatment. The two patients in PR had residual mediastinal disease; one was converted to a CR after Auto-SCT while the other patient was the one who died of multiorgan failure during aplasia after Auto-SCT. Three patients (one T-LBL and two B-LBL) relapsed after 24, 4.5 and 25 months, respectively. Thus six patients experienced treatment failure. The patient with PD had no response to a second line regimen, and the T-LBL patient with relapse received palliative chemotherapy. The two B-LBL patients with relapse 7 months and 18 months after Auto-SCT both achieved a new remission on the Hyper-CVAD regimen followed by conventional myoablative Allo-SCT and are both in second CR 29 and 20 months later, respectively. At present 21 of 25 patients are alive and in CR. With a median follow-up of 98 months (range 1–163) the 5- and 8-year PFS and OS on an intent to treat basis are 76% (95% CI 58–94%) and 84% (95% CI 69–99%), respectively ().
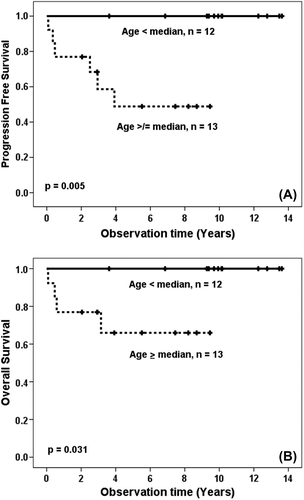
Gender, T/B lineage, lactate dehydrogenase, the International Prognostic Index score for aggressive NLH or extra-nodal involvement did not affect PFS or OS. Age was the only factor which showed a significant impact on outcome. All patients belonging to the younger age group (below the median age of 33 years) are alive and disease-free at last follow-up compared to a PFS and OS of 54% and 69%, respectively, for patients above the median age (p < 0.005 and p < 0.031, ).
Discussion
The present report describes a single centre experience in the treatment of adult patients diagnosed with T- (19 patients) and B- (6 patients) lineage LBL. The median age of patients in our study is somewhat higher than in other studies (33 vs. 25–28 years) [Citation15–17, Citation24,Citation25]. As expected there is a male predominance of 3:2. The patient characteristics are otherwise comparable to previous reports [Citation14,Citation16,Citation17,Citation24,Citation25]. Patients received ALL-like induction an early consolidation chemotherapy, followed up with a late consolidated with Auto-SCT. The great majority of patients had radiotherapy after Auto-SCT to involved sites at diagnosis in the mediastinum, skeleton or testicles. The reported 5- and 8-year PFS and OS of 76% and 84%, respectively for this relatively rare entity compares favorably with similar published data from other institutions [Citation13,Citation14,Citation16,Citation17,Citation24–28] and contributes to the literature on this subject. Only one patient with T-LBL progressed during induction/early consolidation chemotherapy while one patient died from TE during induction. The remaining patients obtained a radiological CR (21 patients) or a PR (2 patients) prior to Auto-SCT. One of the two patients in PR before Auto-SCT died from multiorgan failure during aplasia following Auto-SCT while the other achieved a CR after Auto-SCT and prior to radiotherapy.
CNS prophylaxis is considered mandatory for LBL patients, either given as chemoprevention, as CNS axis irradiation or both [Citation16]. We intensified the CNS prophylaxis compared to the original Hammersmith regimen, and assumed that the BEAM regimen also would contribute to CNS lymphoma protection [Citation20]. Whereas three patients (12%) had CNS involvement at diagnosis, only one of these was treated with CRT. No CNS relapses were observed. In the study by Thomas et al., eight administrations of triple i.t. chemotherapy was given together with the hyper CVAD regimen followed by maintenance chemotherapy [Citation25]. Only 3% (1/33) of the patients had isolated CNS relapse. We accordingly consider it safe to omit CRT. For patients with CNS involvement at diagnosis one might consider to use a high dose regimen like the BU-thiothepa combination instead of BEAM because of reported enhanced CNS protection [Citation29,Citation30].
There is no consensus whether to use maintenance chemotherapy or late consolidation with Auto-SCT after induction chemotherapy and early consolidation in LBL. Maintenance chemotherapy for patients with T-LBL using the German Multicenter Group for adult ALL protocol (GMALL) showed a 7-year OS of 51% while the MD Anderson group reported a 3-year OS of 70% [Citation16,Citation25]. However, a report from the EBMT register showed a 6-year OS of 63% for patients auto-transplanted in first remission [Citation7]. The subsequent and only randomized study was conducted by the EBMT group, comparing Auto-SCT consolidation with maintenance chemotherapy. This study was terminated early due to low accrual rate [Citation14]. However, the results from this showed a trend for PFS benefit in favor of Auto-SCT (hazards ratio = 0.55; 95% confidence interval [CI], 0.29 to 1.04; p = 0.065), but no difference in OS, the latter possibly explained by a cross over in the maintenance arm for patients achieving a second remission. The most recent series of patients with T-LBL treated with a NHL/ALL regimen with “fast track” to Auto-SCT consolidation was reported from British Columbia, showing an impressive 4-year OS and EFS of 72% and 68%, respectively [Citation24]. The results from our T-LBL cohort compare favorably to this and other previous reports in regard to both PFS and OS.
Another controversy for T-LBL concerns the use of mediastinal RT. In our cohort of T-LBL, 93% of eligible patients with an initial mediastinal mass received RT. No local relapse was observed. In the above mentioned German report, 84% of patients underwent mediastinal RT to 24 Gy [Citation16]. Nevertheless, 47% of relapses occurred in the mediastinum. The high number of relapses might have been due to less intensive consolidation therapy, and/or an inadequate radiation dose. As a consequence of these result the authors discussed whether to increase the dose of radiation to 36 Gy [Citation16]. Dabaja and colleagues reported on 47 patients treated with different induction regimes. Of 43 patients in CR after induction, 44% (19 patients) received mediastinal RT, (26–39 Gy). There were no mediastinal relapses for patients receiving RT compared to 33% (8/24) in the group who did not receive RT. Patients who had been treated with more intensive chemotherapy seemed to benefit more from mediastinal RT. Hence, patients who achieve a good remission may get additional benefit from local RT [Citation15]. Cortelazzo et al. report on a risk-adapted strategy where RT was omitted for patients in CR after induction and early consolidation chemotherapy. Patients were further assessed for MRD, and MRD pos patients underwent Auto-SCT. This resulted in a disease-free survival and OS of 77% and 72%, respectively and only 7% mediastinal relapses in non-irradiated patients (24 Gy) [Citation17]. Mediastinal RT combined with chemotherapy is associated with an increased risk of late sequelae, most notably cardiovascular disease and secondary malignancies [Citation31]. Hence, it would be of importance to be able to select the subgroup of patients who would not benefit from RT, as suggested by Cortelazzo and colleagues [Citation17]. FDG-PET/CT might become a useful tool to identify patients who achieve an early FDG-PET negative residual lesion in the mediastinum and therefore might not be in need of RT.
We encountered a number of serious side effects during this intensive multimodal treatment. Altogether eight patients (32%) were diagnosed with TE, one of them being fatal. Increased incidence of TE during ALL induction chemotherapy has been attributed to L-asparaginase [Citation32]. One could consider using low molecular weight heparin for thrombosis-prophylaxis and possibly delay insertion of central venous lines till after L-asparaginase therapy [Citation32]. L-asparaginase is also known to cause disturbances in liver function and we did observe five cases of transiently elevated liver enzymes.
There was a high incidence of both documented bacteremia and opportunistic infections during treatment, but all were treated successfully. We advocate prophylaxis against HSV, PCP and fungal infection with Candida species.
Three patients (aged 26, 30 and 32 years) developed osteonecrosis, a side effect often observed in adolescents and young adults treated for LBL/ALL. Prolonged steroid medication is the most likely cause. In a recent randomized study, a statistically lower incidence was reported when steroids were given days 0–6 and 14–20 instead of continuously during the same time period [Citation33].
Some of the youngest female patients seemed to recover from a temporary drop in reproductive fertility, but for most of the women in their 30s or older, the treatment resulted in premature menopause. Maintenance therapy instead of Auto-SCT might be an alternative for the patients who cannot accept this risk.
In conclusion, we report a high cure rate for LBL patients treated with an ALL-like induction and early consolidation regimen followed by Auto-SCT for responding patients and local radiotherapy for patients with initial mediastinal mass, skeletal or testicular lesions. Our data support this treatment strategy, although the size of the study population is rather small. In spite of the fact that the majority of patients achieve permanent disease control and cure, the treatment-related morbidity is still a matter of concern. Improvements by risk-adapted strategies may be possible by applying MRD testing and FDG-PET/CT in order to select patients who do not benefit from RT and/or Auto-SCT. Further studies to address these questions are warranted and due to the low incidence of these malignancies multi-center collaborations are needed.
Acknowledgments
Thanks to patients and staff at the Norwegian Radium Hospital.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. Geneva: World Health Organization; 2008.
- Rampal R, Levine RL. Leveraging cancer genome information in hematologic malignancies. J Clin Oncol 2013;31: 1885–92.
- Nathwani BN, Diamond LW, Winberg CD, Kim H, Bearman RM, Glick JH, et al. Lymphoblastic lymphoma: A clinicopathologic study of 95 patients. Cancer 1981;48:2347–57.
- Bouabdallah R, Xerri L, Bardou VJ, Stoppa AM, Blaise D, Sainty D, et al. Role of induction chemotherapy and bone marrow transplantation in adult lymphoblastic lymphoma: A report on 62 patients from a single center. Ann Oncol 1998;9:619–25.
- Jost LM, Jacky E, Dommann-Scherrer C, Honegger HP, Maurer R, Sauter C, et al. Short-term weekly chemotherapy followed by high-dose therapy with autologous bone marrow transplantation for lymphoblastic and Burkitt's lymphomas in adult patients. Ann Oncol 1995;6:445–51.
- Milpied N, Ifrah N, Kuentz M, Maraninchi D, Colombat P, Blaise D, et al. Bone marrow transplantation for adult poor prognosis lymphoblastic lymphoma in first complete remission. Br J Haematol 1989;73:82–7.
- Sweetenham JW, Liberti G, Pearce R, Taghipour G, Santini G, Goldstone AH. High-dose therapy and autologous bone marrow transplantation for adult patients with lymphoblastic lymphoma: Results of the European Group for Bone Marrow Transplantation. J Clin Oncol 1994;12: 1358–65.
- Santini G, Coser P, Chisesi T, Porcellini A, Sertoli R, Contu A, et al. Autologous bone marrow transplantation for advanced stage adult lymphoblastic lymphoma in first complete remission. Report of the Non-Hodgkin's Lymphoma Cooperative Study Group (NHLCSG). Ann Oncol 1991;2(Suppl 2):181–5.
- Colgan JP, Andersen J, Habermann TM, Earle JD, O’Connell MJ, Neiman RS, et al. Long-term follow-up of a CHOP-based regimen with maintenance therapy and central nervous system prophylaxis in lymphoblastic non-Hodgkin's lymphoma. Leuk Lymphoma 1994;15:291–6.
- Morel P, Lepage E, Brice P, Dupriez B, D’Agay MF, Fenaux P, et al. Prognosis and treatment of lymphoblastic lymphoma in adults: A report on 80 patients. J Clin Oncol 1992;10:1078–85.
- Anderson JR, Wilson JF, Jenkin DT, Meadows AT, Kersey J, Chilcote RR, et al. Childhood non-Hodgkin's lymphoma. The results of a randomized therapeutic trial comparing a 4-drug regimen (COMP) with a 10-drug regimen (LSA2-L2). N Engl J Med 1983;308:559–65.
- Coleman CN, Picozzi VJ, Jr., Cox RS, McWhirter K, Weiss LM, Cohen JR, et al. Treatment of lymphoblastic lymphoma in adults. J Clin Oncol 1986;4:1628–37.
- Slater DE, Mertelsmann R, Koziner B, Higgins C, McKenzie S, Schauer P, et al. Lymphoblastic lymphoma in adults. J Clin Oncol 1986;4:57–67.
- Sweetenham JW, Santini G, Qian W, Guelfi M, Schmitz N, Simnett S, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: Results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol 2001;19:2927–36.
- Dabaja BS, Ha CS, Thomas DA, Wilder RB, Gopal R, Cortes J, et al. The role of local radiation therapy for mediastinal disease in adults with T-cell lymphoblastic lymphoma. Cancer 2002;94:2738–44.
- Hoelzer D, Gokbuget N, Digel W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T- lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002;99:4379–85.
- Cortelazzo S, Intermesoli T, Oldani E, Ciceri F, Rossi G, Pogliani EM, et al. Results of a lymphoblastic leukemia-like chemotherapy program with risk-adapted mediastinal irradiation and stem cell transplantation for adult patients with lymphoblastic lymphoma. Ann Hematol 2012;91:73–82.
- Sweetenham JW, Mead GM, Whitehouse JM. Adult lymphoblastic lymphoma: High incidence of central nervous system relapse in patients treated with the Stanford University protocol. Ann Oncol 1992;3:839–41.
- Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971;31:1860–1.
- Marcus RE, Catovsky D, Johnson SA, Gregory WM, Talavera JG, Goldman JM, et al. Adult acute lymphoblastic leukaemia: A study of prognostic features and response to treatment over a ten year period. Br J Cancer 1986;53:175–80.
- Evensen SA, Brinch L, Tjonnfjord G, Stavem P, Wisloff F. Estimated 8-year survival of more than 40% in a population-based study of 79 adult patients with acute lymphoblastic leukaemia. Br J Haematol 1994;88:88–93.
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: A randomised trial. Lancet 2002;359:2065–71.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc 1958;53:457–81.
- Song KW, Barnett MJ, Gascoyne RD, Chhanabhai M, Forrest DL, Hogge DE, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol 2007;18:535–40.
- Thomas DA, O’Brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004;104:1624–30.
- Bernasconi C, Brusamolino E, Lazzarino M, Morra E, Pagnucco G, Orlandi E. Lymphoblastic lymphoma in adult patients: Clinicopathological features and response to intensive multiagent chemotherapy analogous to that used in acute lymphoblastic leukemia. Ann Oncol 1990;1:141–6.
- Hunault M, Truchan-Graczyk M, Caillot D, Harousseau JL, Bologna S, Himberlin C, et al. Outcome of adult T-lymphoblastic lymphoma after acute lymphoblastic leukemia-type treatment: A GOELAMS trial. Haematologica 2007;92:1623–30.
- Jabbour E, Koscielny S, Sebban C, Peslin N, Patte C, Gargi T, et al. High survival rate with the LMT-89 regimen in lymphoblastic lymphoma (LL), but not in T-cell acute lymphoblastic leukemia (T-ALL). Leukemia 2006;20: 814–9.
- Ferreri AJ. How I treat primary CNS lymphoma. Blood 2011;118:510–22.
- Ferreri AJ, Crocchiolo R, Assanelli A, Govi S, Reni M. High-dose chemotherapy supported by autologous stem cell transplantation in patients with primary central nervous system lymphoma: Facts and opinions. Leuk Lymphoma 2008;49:2042–7.
- Wethal T, Lund MB, Edvardsen T, Fossa SD, Pripp AH, Holte H, et al. Valvular dysfunction and left ventricular changes in Hodgkin's lymphoma survivors. A longitudinal study. Br J Cancer 2009;101:575–81.
- Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-Asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia 2013; 27:553–9.
- Mattano LA, Jr., Devidas M, Nachman JB, Sather HN, Hunger SP, Steinherz PG, et al. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: Results from the CCG-1961 randomised cohort trial. Lancet Oncol 2012;13:906–15.