To the Editor,
Most patients (70–80%) with Hodgkins lymphoma (HL) achieve a long-term complete remission (CR) after a standard ABVD therapy, but some, to be cured, require more intensive, but also more toxic treatment, such as escalated BEACOPP [Citation1,Citation2]. As a result, the main task for the clinician is to tailor the therapy to an individual patient's needs balancing between efficacy and toxicity [Citation3]. A useful tool for the identification of patients with high risk of ABVD treatment failure appears to be an interim positron emission tomography combined with computed tomography (PET/CT). Normalization of PET/CT is seen in nearly 80% of HL patients already after two cycles of ABVD [Citation2]. Early identification of patients who are resistant to standard ABVD treatment would allow for early treatment intensification. Assuming high efficacy of the escBEACOPP, this regimen would be treatment of choice for such intensification. However, the data reporting the feasibility and efficacy of such an approach are scarce. There is only one Italian collaborative retrospective analysis reporting on treatment intensification with BEACOPPesc in 23 patients with positive interim PET during ABVD therapy [Citation4]. Here we report the results of BEACOPP intensification in 10 patients with positive interim PETs during ABVD treatment who have either clinical evidence of disease progression or requested such treatment change based on the two subsequent interim PET results.
The presented 10 patients (8 men and 2 women), mean age 35 (21–61) years initially had been enrolled to the observational study run by the Polish Lymphoma Research Group that aimed at evaluating the prognostic role of PET/CT performed after the first (PET-1) ABVD cycle. If a PET-1 result was positive, a PET-2 was performed after the second cycle. All PET/CT scans were performed at the same institution and were assessed according to the five-point scale/Deauville criteria by dedicated nuclear medicine specialists trained in interim PET assessment. No treatment change was planned because of PET results; however, in case of clinical evidence of disease progression or a lack of response to ABVD chemotherapy, such as physical examination and contrast enhanced CT, treatment modification was allowed. All 10 patients being informed about the PET results and their prognosis decided to withdraw from the observational study and requested treatment change. In five of those patients in addition to the positive interim PETs, there was evidence, based on physical examination and classical contrast enhanced CT, for disease progression, or a lack of response to ABVD treatment. In one of those patients, the presence of active disease was confirmed by histopathology examination. All 10 patients were offered six cycles of escalated BEACOPP. The clinical characteristics of patients are listed in .
Table I. Clinical patients’ characteristics.
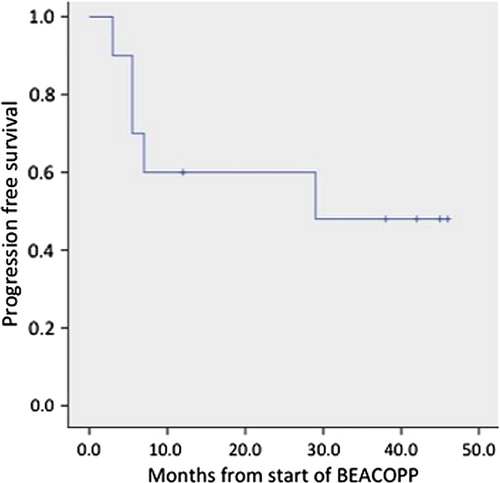
All patients, except one, completed the six scheduled cycles of BEACOPPesc chemotherapy. One patient, a 61-year-old woman, died during the third cycle, being in pancytopenia due to sepsis. Two patients (UPN 794001 and 794416, ) did not respond and had clearly positive PET after BEACOPP completion and were offered salvage radiotherapy with extended fields. These patients fortunately remain in complete response (CR) for now 18 months. Seven patients achieved CR confirmed by negative end therapy PET (score 1, 2 and 3). One of those patients (UPN573051) received consolidation radiotherapy to the initially bulky lesions that presented minimal residual uptake. This patient remains in CR. During the follow-up two patients (UPN658855 and UPN634236) relapsed, one four months, and the other 26 months after last BEACOPP administration. The early relapse occurred in the initially involved sites. The late relapse, however, happened in a completely new site. Both patients were treated with second line treatment (IGEV) without anatomic and metabolic response and therefore were subjected to salvage radiotherapy. Overall at a median observation period of 26 (3–49) months the probability of two-year progression free survival (PFS) was 60% ().
Table II. The results of interim PETs and clinical course.
Our report consists of a highly selected population of patients most with advanced disease, and bulky lesions, extranodal involvement, and presence of B symptoms with two subsequent positive interim PET results. Although in all patients 18-FDG uptake after one ABVD cycle compared to baseline PET (data not shown) decreased, subsequent PET-2 results either showed no further decrease (five patients), or the decrease of 18-FDG uptake was insufficient to consider a patient as responding to ABVD therapy. Additionally, some patients had clinical signs of disease persistence or progression either documented by physical examination or contrast enhanced CT. Most of the patients (70%) responded to BEACOPP and achieved PET negativity. Two relapses – one early, the other more than two years after BECAOPP completion – question the response stability. Despite the small number of patients our results (50% of patients in CR with 60% PFS at two years) are comparable with a first retrospective report on early BEACOPP intensification in patients with positive PET after two ABVD cycles where 15 of 23 patients (65%) remained in CR after BEACOPP intensification (four escalated and four baseline) with two-years failure free survival 65% [Citation4]. Also, the first interim results of prospective clinical trials of BEACOPP intensification show a complete response in BEACOPP arms in 23 of 32 patients (72%) [Citation5]. Taken together, these results indicate that escalated BEACOPP intensification is effective in about two thirds of ABVD resistant patients.
The two relapses after BEACOPP completion were resistant to third line IGEV treatment. This is consistent with recent observation of limited efficacy of autologous transplantation in patients with BEACOPP failure [Citation6]. Those observations point towards a statement that BEACOPP resistance identify chemo-resistant patients that should be offered alternative approaches such as salvage radiotherapy if possible.
It is unquestionable that our patients need to be fully informed of the various pathways to cure, their likelihood of success, and short- and long-term toxicity costs [Citation3]. The results of BEACOPP intensification presented here was a consequence of such attitude. The patients who agreed to participate in the observational study were fully informed of the results of interim PETs and their prognosis. As a result 10 presented patients had withdrawn their consent to continue ABVD treatment. Thus, our study indicates that physicians who perform interim PET outside clinical trials must be aware of its inevitable consequence; having done the interim PET, the treating physician must respond to a patient query related to the PET result. However, accepting early assessment of therapeutic efficacy by PET/CT as the standard procedure requires further clinical evidence as of yet. Nevertheless, there are grounds to believe [Citation5] that the interim PET will become an essential and obligatory element in the individualization of the treatment in HL patients in the initial phase of standard ABVD therapy. Despite the unintended design of our report, we can conclude that six cycles of escalated BEACOPP are feasible, relatively safe, and effective in HL patients not responding to ABVD. The patients resistant to BEACOPP intensification are at high risk of being resistant to other chemotherapy regimens and require an alternative treatment approach.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med 2003;348:2386–95.
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-d- glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: A report from a joint Italian-Danish study. J Clin Oncol 2007;25:3746–52.
- Longo DL. Reply to P. Borchmann et al. J Clin Oncol 2013;31:3046.
- Gallamini A, Patti C, Viviani S, Rossi A, Fiore F, Di Raimondo F, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with an interim-PET positive after two ABVD courses. Br J Haematol 2011;152:551–60.
- Gallamini A, Rossi A, Patti C, Picardi M, Di Raimondo F, Cantonetti M, et al. Early treatment intensification in advanced-stage high-risk Hodgkin lymphoma (HL) patients, with a positive FDG-PET scan after two ABVD courses – first interim analysis of the GITIL/FIL HD0607 clinical trial. ASH Annual Meeting Abstracts 2012;120:550.
- Wannesson L, Bargetzi M, Cairoli A, Cerutti A, Heim D, Hess U, et al. Autotransplant for Hodgkin lymphoma after failure of upfront BEACOPP escalated (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone). Leuk Lymphoma 2013;54:36–40.