To the Editor,
The liver is a common site of metastases from many solid organ malignancies. In patients with oligometastatic liver disease, systemic therapy, generally in combination with surgical resection, is considered standard of care. In colorectal cancer, this combined approach has improved five-year survival rates to 50–60% [Citation1]. However, only 10–25% of patients will be initially appropriate for surgery, due to unfavourable disease distribution or co-morbidities [Citation2]. For the remainder, a number of non-surgical invasive and non-invasive ablative techniques may be used as alternative modalities to achieve local control. Of these, partial liver irradiation (PLI), (also known as stereotactic body radiation therapy, SBRT) represents an attractive non-invasive option, with a rapidly expanding body of evidence supporting its use. High rates of LC (60–90% at two years), together with an acceptable toxicity profile (< 10% G3 toxicity) have led to it being increasingly integrated into the therapeutic pathway [Citation3,Citation4]. Here it competes with alternative local options such as radiofrequency ablation (RFA), cryotherapy, microwave, selective internal radiation therapy (SIRT) and trans-arterial chemoembolisation (TACE).
Small, favourably located lesions may be successfully ablated by a number of these techniques with similar local control outcomes, as demonstrated in a recent matched pair analysis of RFA compared to SBRT [Citation5]. More challenging is the management of large volume lesions, often situated adjacent to critical structures, where many of these techniques are unsuitable or are known to result in inferior LC results. In this sub-group significantly less reported experience exists, both in the use of PLI and alternative strategies.
We have approached the safe treatment of large lesions with SBRT by individualising the radiation dose, using a normal tissue complication probability (NTCP) model. This effectively allows individualisation of tumour dose according to the modelled risk of radiation-induced liver disease (RILD) for each patient, a toxicity of particular concern when treating large lesions. The prescribed dose is dependent on the volume of normal liver tissue exposed to radiation and has been previously described [Citation6].
This report evaluates the safety and efficacy of a biologically driven prescription method for moderately hypofractionated RT strategy, in a heavily pretreated group of patients with, in general, larger volume liver metastases.
Patients and methods
Patient selection
This was an institutionally approved retrospective review of patients who had undergone PLI between December 2006 and December 2011 at The Royal Marsden Hospital. Eligible patients were retrospectively identified from the hospital electronic patient record system. Due to heterogeneity in the dose and fractionation schedules used during this time period, we chose to limit this analysis to patients receiving PLI in a 10 fraction regime, reflecting the most commonly utilised fractionation for treating larger volume lesions.
Patient suitability for PLI was confirmed in a Hepatobiliary Multidisciplinary meeting (MDM). All patients had technically unresectable disease or were deemed unsuitable for surgery, either due to co-morbidities or the presence of extrahepatic disease (EHD). The initial cohort of patients were treated within the context of a single centre Phase 1 trial, with the aim of establishing the safety of PLI in CRC patients who had exhausted all standard systemic therapeutic options. Subsequently, this protocol was expanded to include the treatment of patients with MLD from any primary site. Any prior therapy was allowed, although in general patients were referred with treatment refractory disease or if further chemotherapy was felt inappropriate. Patients undergoing PLI for primary hepatobiliary tumours were excluded from this analysis. Data on prior systemic and hepatic directed therapies prior to PLI was recorded.
Eligible patients had ≥ 700 cm3 of uninvolved liver and adequate baseline organ function. No more than three discrete lesions were permitted with no upper limit on tumour size, if the planning constraints could be met. Patients were required to have a performance status ≤ 2 at baseline and an estimated life expectancy > 3 months.
Radiotherapy planning and treatment
Radiotherapy was computed tomography (CT) planned using a highly conformal multi field technique (Supplementary Figure 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/ 0284186X.2013.862595). Active Breath Control (ABC) or four-dimensional (4D) CT was used to account for respiratory motion. An isotropic 5 mm margin was added to the gross tumour volume (GTV) to form the clinical target volume (CTV). The CTV to planning target volume (PTV) margin was patient specific dependent on the immobilisation device (minimum 5 mm). The organs at risk (OAR) defined and normal tissue planning constraints are listed in Supplementary Table I (to be found online at http://informahealthcare.com/doi/ abs/10.3109/0284186X.2013.862595).
The prescribed dose was individualised according to the effective liver volume irradiated, Veff, using a Lyman-Kutcher-Burman (LKB) NTCP model, as previously described by other authors [Citation7]. Dose was prescribed to the 100% with a minimum of 90% of the prescribed dose covering the PTV. Treatment was delivered on a consecutive day regime, treating 5 fractions per week. For the purposes of analysis, a biologically effective dose (BED) was calculated using the formula BED = (Prescription dose)* [1+ (dose per fraction/α/β)] assuming an α/β ratio of 10 and no time factor correction.
Follow-up, study end-points and statistical analysis
Follow-up consisted of clinical, radiological, and biochemical assessments every 3–6 months. Treatment response was determined using standard RECIST criteria (version 1.1) [Citation8]. If more than one lesion was treated per patient, each lesion was evaluated independently. Kaplan-Meier analysis for local control (LC), progression free survival (PFS) and overall survival (OS) was performed using SPSS v21. Median follow-up was calculated using the inverse Kaplan-Meier method.
Cox regression univariate analysis was performed to identify factors predictive for improved local control (lesion-based analysis) and OS. Spearman's rank correlation co-efficient was calculated to assess the relationship between GTV size and BED (total GTV volume per patient if multiple lesions). Toxicities were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 [Citation9].
Results
Patient characteristics
Thirty-four patients with 46 discrete lesions (range 1–3 per patient) were treated in the time period. The baseline patient characteristics are summarised in Supplementary Table II (to be found online at http://informahealthcare.com/doi/abs/10.3109/ 0284186X.2013.862595) The median age was 71 years (range 44–85). Forty percent had received liver-directed intervention and the majority of patients (80%) had received prior chemotherapy, with a median of two lines of systemic therapy prior to PLI (range 0–4). The seven patients who did not receive prior systemic therapy were considered to be medically unfit. The commonest primary site was colorectal cancer (n = 27) followed by breast (n = 4). Thirty-five percent of patients had extra-hepatic diseae present at the time of PLI.
Lesion characteristics
Forty-six discrete lesions were treated in 42 GTV volumes (in four patients, two adjacent lesions were combined into a single GTV for the purposes of radiotherapy planning). The median GTV volume as measured in cubic centimetres (cm3) on the planning scan was 73.1 (range 2.0–614.0). Median GTV size as measured in any axis was 5.1 cm (range 2.1–13.5). Lesion characteristics are summarised in Supplementary Table III (to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.862595).
Dose
All patients were treated in a 10 fraction regime. The prescribed dose ranged from 30 Gy (BED10 = 39) to 60 Gy (BED10 = 96).
Toxicity
Overall the treatment was well tolerated with little toxicity recorded. A single patient had an acute (defined as ≤ 3months) Grade 3 toxicity in the form of an asymptomatic increase in activated partial thromboplastin time (APTT). This resolved with conservative management within four weeks of treatment. This patient had been heavily pre-treated, having previously undergone a right hemi-hepatectomy, three RFA procedures, and two lines of systemic chemotherapy. Acute grade 1 toxicity was common, being experienced by 70% of patients, most commonly fatigue (n = 11) followed by an asymptomatic alkaline phosphatase rise (n = 5) and nausea (n = 4). No late toxicity was observed.
Clinical outcomes
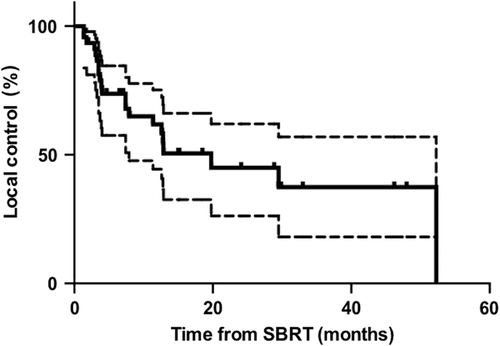
Local control, progression free survival and overall survival. At a median follow-up of 15 months (range 1.9–70.6), 21 of 46 (45.7%) lesions had progressed locally. Median time to local progression on a lesion-based analysis was 19.8 months (range 1.4–52.3). One- and two-year local control rates were 61.8% (95% CI 46.3–77.3) and 45.0% (95% CI 26.6–63.4), respectively (). On a patient-based analysis, local control results were similar with a one- and two-year local control of 64.0% (95% CI 48.5 –79.5) and 45.0% (95% CI 26.6–63.4), respectively. Overall 16/34 patients experienced local progression during the period of follow-up.
In total 26/34 (76%) patients had experienced distant progression at last follow-up. The commonest sites of progression were lung (n = 13) followed by out-of-field progression in the liver (n = 9). However, the majority of patients (59%) were fit enough to go onto receive further active treatment, most commonly in the form of palliative chemotherapy (n = 19). Median time to distant progression was 4.8 months (range 1.2–52.3). Overall PFS was 29.4% (95% CI 14.1–43.7) and 16.1% (95% CI 3.2–29.0) at one and two years, respectively, reflecting the high rates of distant progression. Median OS was 14.5 months (range 1.2–52.3). One and two year OS rates were 59.8% (95% CI 42.9—76.7) and 38.0% (95% CI 20.1–55.8), respectively (Supplementary Figure 2, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.862595).
Univariate analysis of predictive factors for LC and OS. On univariate analysis for local control, GTV size ≤ 60 cm3 and BED10 > 50 were predictive for improved time to local failure. GTV size ≤ 60 cm3 and the absence of extra hepatic disease were predictive for improved OS, as summarised in . BED > 50 was of borderline statistical significance (p = 0.056) for improved OS.
Table I. Univariate analysis of predictive factors for local control and overall survival. Figures in bold represent hazard ratios with statistically significant p-values (p < 0.05).
There was a significant negative correlation between GTV size and BED using a Spearman's rank test (correlation co-efficient −0.38, p = 0.01). However, the difference in mean BED in those with a single largest GTV volume > 60 cm3 compared to those with a GTV ≤ 60 cm3 did not reach statistical significance using an independent samples t-test (p = 0.061).
Discussion
Achieving local control in patients with large volume liver metastases remains a challenging clinical problem. Many local therapies are technically unsuitable for treating large lesions. Radiofrequency ablation (RFA), currently the most established local therapy option, results in significantly inferior LC rates in lesions > 3 cm [Citation10]. Similarly microwave and cryotherapy use is reserved for small lesions. This study demonstrates that SBRT, using this approach of individualised dose PLI, is a well-tolerated and effective option for achieving LC in this patient group.
Published local control rates in the literature for liver SBRT vary from 57– 100% at 1–2 years [Citation11–19]. The one-year LC rate of 61.8% in this series compares to the 67% one year LC in a recent pooled meta-analysis of 65 patients (102 lesions) with MLD from colorectal cancer treated with SBRT [Citation20]. The observed LC whilst being at the lower end of the published range is likely to reflect a combination of relatively conservative BED and larger lesion size. The median GTV volume was larger than in most reported series with only one previous series reporting such high median tumour volumes [Citation15]. On univariate analysis, GTV size ≤ 60 cm3 and BED > 50 were found to correlate with improved LC. Multivariate analysis was not performed due to the relatively small sample size. However, these factors are likely to be a function of one another, rather than independent variables, with the statistically significant correlation between GTV size and BED to be expected given the methodology used for dose prescription.
It is now established that dose is an important determinant of LC, with a dose response relationship being observed by several authors [Citation20–23]. Indeed, in the pooled meta-analysis reported by Chang et al., GTV volume, as a factor independent of dose, was not predictive of local control on multivariate analysis [Citation20], a finding recently confirmed by Scorsetti et al. [Citation24]. This suggests that if a threshold dose can be safely delivered without exceeding normal tissue tolerances, tumour size alone may not lead to inferior outcomes. It has been proposed using modelling data, that the dose required to achieve > 90% local control at one year is a BED ≥ 117 (assuming an α/β ratio of 10), significantly higher than the maximum BED of 96 used in our series [Citation20]. However, delivering a BED of this magnitude to large lesions without exceeding normal tissue tolerances is often not achievable, despite the use of advanced radiotherapy techniques. Further work to improve our understanding of the biological response to SBRT and causative mechanisms underlying local failure is therefore needed.
The predictive effect of tumour size on LC in response to SBRT is not clear. Several authors have found local control rates to be inferior in larger volume lesions [Citation14,Citation15]. Potential causative mechanisms include the presence of chemoresistant clonogens which in turn are more likely to be radioresistant, as well as regions of hypoxia within large lesions [Citation25]. In colorectal cancer, tumour hypoxia has been demonstrated to be present heterogeneously throughout resected specimens [Citation26]. Furthermore, van Laarhoven et al. have demonstrated in resected liver metastases with a mean size of 43.9 ± 25.3 mm (range 10–100 mm) [Citation27], areas of chronic hypoxia close to areas of proliferation, raising the possibility of high oxygen consumption alongside limited oxygen supply. The mean pimonidazole-positive fraction was 0.146 and this appears to be higher than in cervix and head and neck malignancies. The use of a more protracted dose fractionation regime may therefore have several radiobiological advantages when treating large lesions. The smaller dose per fraction reduces the risk of late normal tissue damage for a given biological dose. Additionally, the extended treatment time provides a window of time in which to both evaluate, and potentially then modify, the tumour microenvironment during radiation. The two-week treatment period provides an opportunity to integrate novel functional imaging techniques, such as F-MISO and FLT PET, into the treatment pathway. Imaging during the first few fractions of treatment may potentially help us to characterise better the tumour environment, and identify individual factors, so intervention in terms of biological modulation (i.e. using a hypoxia modifying or anti-proliferative agent) or adaptive dose boost deposition can be applied. This would allow PLI to be personalised, not only dosimetrically, but also biologically according to the individual tumour microenvironment. Staehler et al. has demonstrated that this approach is feasible [Citation28]. This alternative strategy to improve LC rates is particularly relevant in the treatment of large volume metastases, where dose escalation is not feasible.
Conclusion
Partial liver irradiation is a safe and effective treatment for the management of bulky metastatic liver disease, in a heavily pre-treated patient population. In patients with large volume metastases, efficacy is limited by the inability to safely deliver sufficient dose, without exceeding normal tissue tolerances. Further evaluation of strategies to improve the therapeutic index in such patients, such as combining PLI with radiosensitising drugs, are warranted.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.862595
Download PDF (1 MB)Declaration of interest: This work was undertaken in The Royal Marsden NHS Foundation Trust which received a proportion of its funding from the NHS Executive; we acknowledge support from the NIHR Royal Marsden/ICR Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NHS executive. Dr Katharine Aitken was funded by the Cridlan Trust. Dr Maria Hawkins acknowledges funding from the MRC. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jones RP, Malik HZ, Fenwick SW, Poston GJ. Perioperative chemotherapy for resectable colorectal liver metastases: Where now?. Eur J Surg Oncol 2013;39:807–11.
- Hewish M, Cunningham D. First-line treatment of advanced colorectal cancer. Lancet 2011;377:2060–2.
- Hoyer M, Swaminath A, Bydder S, Lock M, Mendez Romero A, Kavanagh B, et al. Radiotherapy for liver metastases: A review of evidence. Int J Radiat Oncol Biol Phys 2012;82: 1047–57.
- Lock MI, Hoyer M, Bydder SA, Okunieff P, Hahn CA, Vichare A, et al. An international survey on liver metastases radiotherapy. Acta Oncol 2012;51:568–74.
- Stintzing S, Grothe A, Hendrich S, Hoffmann RT, Heinemann V, Rentsch M, et al. Percutaneous radiofrequency ablation (RFA) or robotic radiosurgery (RRS) for salvage treatment of colorectal liver metastases. Acta Oncol 2013;52:971–7.
- Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol 2006;45: 856–64.
- Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810–21.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
- Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC).
- Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, 3rd, Dorfman GS, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010;28:493–508.
- Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: Update of the initial phase-I/II trial. Front Radiat Ther Oncol 2004; 38:100–5.
- Mendez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase I-II study. Acta Oncol 2006;45:831–7.
- Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006;45:823–30.
- Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572–8.
- Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009; 27:1585–91.
- Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486–93.
- Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006;45:838–47.
- Katz AW, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 2007;67:793–8.
- van der Pool AE, Mendez Romero A, Wunderink W, Heijmen BJ, Levendag PC, Verhoef C, et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg 2010;97:377–82.
- Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J, et al. Stereotactic body radiotherapy for colorectal liver metastases: A pooled analysis. Cancer 2011;117:4060–9.
- McCammon R, Schefter TE, Gaspar LE, Zaemisch R, Gravdahl D, Kavanagh B. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2009; 73:112–8.
- Rule W, Timmerman R, Tong L, Abdulrahman R, Meyer J, Boike T, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011;18:1081–7.
- Lanciano R, Lamond J, Yang J, Feng J, Arrigo S, Good M, et al. Stereotactic body radiation therapy for patients with heavily pretreated liver metastases and liver tumors. Front Oncol 2012;2:23.
- Scorsetti M, Arcangeli S, Tozzi A, Comito T, Alongi F, Navarria P, et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013;86:336–42.
- Brown JM, Diehn M, Loo BW, Jr. Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys 2010;78: 323–7.
- Goethals L, Debucquoy A, Perneel C, Geboes K, Ectors N, De Schutter H, et al. Hypoxia in human colorectal adenocarcinoma: Comparison between extrinsic and potential intrinsic hypoxia markers. Int J Radiat Oncol Biol Phys 2006;65:246–54.
- van Laarhoven HW, Kaanders JH, Lok J, Peeters WJ, Rijken PF, Wiering B, et al. Hypoxia in relation to vasculature and proliferation in liver metastases in patients with colorectal cancer. Int J Radiat Oncol Biol Phys 2006;64:473–82.
- Staehler M, Haseke N, Stadler T, Nuhn P, Roosen A, Stief CG, et al. Feasibility and effects of high-dose hypofractionated radiation therapy and simultaneous multi-kinase inhibition with sunitinib in progressive metastatic renal cell cancer. Urol Oncol 2012;30:290–3.