Abstract
Non-small cell lung cancer (NSCLC) is associated with poor survival even though patients are treated with curatively intended radiotherapy. Survival is affected negatively by lack of loco-regional tumour control, but survival is also influenced by comorbidity caused by age and smoking, and occurrence of distant metastasis. It is challenging to evaluate loco-regional control after definitive radiotherapy for NSCLC since it is difficult to distinguish between radiation-induced damage to the lung tissue and tumour progression/recurrence. In addition it may be useful to distinguish between intrapulmonary failure and mediastinal failure to be able to optimize radiotherapy in order to improve loco-regional control even though it is not easy to discriminate between the two sites of failure.
Material and methods. This study is a retrospective analysis of 331 NSCLC patients treated with definitive radiotherapy from 2002 to 2011. The patients were treated consecutively at the Department of Oncology, Odense University Hospital, Denmark with at least 60 Gy. All patients were followed in a planned follow-up schedule and no patients were lost for follow-up.
Results. At the time of the analysis 93 patients had loco-regional failure only. Of these patients, 68 had intrapulmonary failure only, one patient had failure in mediastinum only, and 24 patients had intrapulmonary failure as well as mediastinal failure. Of the patients which had lung failure only, 78% had mediastinal involvement at treatment start. The only covariate with significant impact on developing intrapulmonary failure only was gross tumour volume. Median survival for the total group of 331 patients was 19 months. The median survival for patients with intrapulmonary failure only was 19 months, and it was 20 months for the patients with mediastinal relapse.
Conclusion. We conclude that focus should be on increasing doses to intrapulmonary tumour volume, when dose escalation is applied to improve local tumour control in NSCLC patients treated with definitive radiotherapy, since most recurrences are located here.
Lung cancer is one of the most common cancers worldwide and the leading cause of cancer death with approximately 1.3 million estimated deaths a year [Citation1]. Non-small cell lung cancer (NSCLC) represents around 85% of all lung cancer cases. For patients with loco-regionally advanced NSCLC in good performance status the standard of care is definitive chemo-radiotherapy [Citation2–4]. This treatment has evolved over time, especially the radiotherapy in which planning and delivery have been increasingly advanced by the introduction of three-dimensional computed tomography (3D CT) planning, later on 4D CT, and fluorodeoxyglucose-positron emission tomography (FDG-PET) as planning tools. The dose distribution has been increasingly conformal due to modern planning techniques such as intensity- modulated radiotherapy (IMRT) and arc therapies. Overall survival rates, however, remain poor with 30% alive after two years and 10–15% after five years in clinical trials [Citation2,Citation5]. Lack of tumour control affect survival negatively [Citation6,Citation7], but survival is also influenced by comorbidity caused by age and smoking, and occurrence of distant metastasis. Local tumour control at two years is as low as 30% in clinical trials on radiotherapy to loco-regional advanced NSCLC [Citation2,Citation5]. From literature it is, however, difficult to conclude where the failure is positioned. It may be important to discriminate between intrapulmonary failure (T site ± N1) and mediastinal failure (N2/3), in order to improve local control by escalating dose or incorporate surgery as a modality [Citation8].
A sustained effort has been invested to improve local control in NSCLC; concomitant chemotherapy added a statistically significant, but modest improvement on loco-regional control rate in a meta-analysis [Citation2]. Based on experience from other cancer types, i.e. head and neck cancer and cervical cancer [Citation9,Citation10], it could be expected that dose escalation would improve local control. However, severe side effects such as radiation-induced pneumonitis and fistulas of oesophagus or bronchial tree are of concern and have been limiting factors for dose escalation [Citation11]. Side effects such as pneumonitis are among others dependent on the irradiated volume [Citation12] and therefore reduced treatment margins have been attempted in order to reduce the radiated volume by introducing 4D CT and daily online imaging before treatment [Citation13,Citation14].
The possibility to escalate dose for a given patient depends on the location of the target. Targets close to critical organs, e.g. within the mediastinum is more difficult to escalate than tumours in the lung. As shown in a previous planning study, it is possible to dose escalate the part of the tumour situated in the lung to high doses [Citation15]. However, the success of such an approach could depend on the actual location of the local failure. Thus, in the current study the distribution of local failure sites between primary tumour (intrapulmonary) and lymph nodes (mediastinum) or both are investigated.
Material and methods
The current retrospective study included 331 patients with cytological or histological confirmed diagnosis of NSCLC consecutively treated with definitive radiotherapy at the Department of Oncology, Odense University Hospital, Denmark from 2002 to 2011. All patients had fulfilled a radiotherapy course to at least 60 Gy. The majority of the patients were staged in mediastinum with mediastinoscopy or endo- bronchial ultrasound. During 2004 PET-CT was gradually introduced as part of the staging procedure. Treatment and planning has evolved over time. In this time period induction chemotherapy with a platin doublet was standard of care for patients in good performance status. Concomitant chemo-radiation was not a standard of care early in the time period, but weekly docetaxel has been an option in selected cases and in 2007 it became standard of care for patients in good performance status. Since 2009 carboplatin and vinorelbine has been the standard concomitant regimen for patients with good performance status. Patients were treated with different radiotherapy dose levels: 60 Gy/30 F, 66 Gy/33 F, and 80 Gy/35 F, 5 fractions a week. All treatments were 3D planned and treated with at least three beams. In 2006 IMRT was introduced and became gradually the standard of care until 2010 when volume-modulated arc therapy (VMAT) was introduced. Since 2007 treatment planning was based on the mid-ventilation phase obtained from a 4D CT. No elective node irradiation was performed, and all beams were treated at each fraction. In order to reduce the effect from divergence in treatment strategies, two time periods was introduced as covariates: early (2002–2006) and late (2007–2011).
Patients were registered prospectively. Additional data was obtained from patient charts. No patients were lost for follow-up. A CT scan was performed four weeks after termination of radiotherapy as baseline for further follow-up. The follow-up schedule was every third months in two years and hereafter every sixth month for another three years. Medical history, clinical examination, and chest x-ray was conducted at each visit. In case recurrent disease was suspected either clinical or from chest x-ray, a CT scan was performed. The CT scan was always evaluated by a radiologist often in collaboration with a clinical oncologist. If possible, a biopsy was taken from a suspect lesion.
In order to test whether any covariates had significant impact on developing intrapulmonary failure, only different covariates (gender, median age, performance status, histology, neoadjuvant chemotherapy, concomitant chemotherapy, T stage (T3–4), mediastinal lymph node involvement at treatment start, median GTV, dose, and treatment period) were tested in a logistic regression analysis.
In order to test the model for size of GTV this volume was obtained from planning system. Tumour and node were staged according to AJJC edition 5 [Citation16].
Distant failure was registered if metastases were observed outside mediastinum and/or outside the primary affected lobe of the lung, in this respect tumour in contralateral lung or supraclavicular lymph nodes without mediastinal involvement was regarded as distant failure. A relapse was registered if significant growth of tumour according to RECIST criteria was observed, if previous infiltrative changes became more solid or if tumour became visible after complete response. Progression of intrapulmonary lymph nodes was considered as intrapulmonary progression. Mediastinal failure was registered if the CT scan report described new enlarged lymph nodes in mediastinum, although it was not always pathological confirmed. “No mediastinal nodal failure” was registered in patients if the report indicated “no visible lymph nodes” in mediastinum. The same was true if the CT description indicated unchanged visible, but not enlarged lymph nodes.
Baseline was the date of radiotherapy start. Date of recurrence was the date of which the first radiographic examination indicated a relapse that may have been verified by a later examination.
Data collection was ended March 2013.
Statistics
Time to recurrence was calculated from first day of radiotherapy. Time to local recurrence was analysed using Kaplan-Meier and Log-rank test.
Logistic regression was performed to analyse the risk of developing intrapulmonary recurrence only from the population. Possible prognostic factors were included in the analyses and a stepwise backward method was used for selection of covariates in the risk of developing local recurrence. Confidence intervals (CI) at 95% for odds risk (OR) were calculated. χ2 or Fischer's exact test were used for test of proportions and Mann-Whitney U-test to compare medians. p-Values less than 0.05 were considered statistically significant. All statistical analyses were performed using Medlog (www.medlog.com).
Results
Baseline characteristics of the patient population are summarised in . At time of radiotherapy commencement 242 patients had mediastinal lymph node involvement. The remaining part of the population was either inoperable due to T stage or comorbidity. Follow-up time (from start of radiotherapy until date of study closure) was at minimum 14.4 months and median 66 months.
Table I. Baseline characteristics of the patient population (N = 331).
When data collection was ended, 98 patients were either alive or had died without relapse. They were excluded from further analysis on pattern of recurrence. This left 233 for further study of pattern of relapse. Of the remaining population 89 patients had distant failure and 51 of the patients had distant failure as well as local failure. This left 93 patients for further analyses on pattern of loco-regional failure only.
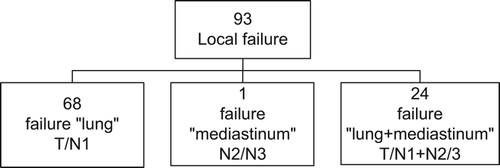
Of the 93 patients with loco-regional failure only, the majority (68 patients) had intrapulmonary failure only, one patient had mediastinal failure only, and 24 patients had mediastinal failure as well as intrapulmonary failure (). One patient had mediastinal failure solely and was grouped with the group of patients who had failure in the lung as well as mediastinum. This led to two groups used for analysis of pattern of local failure: 1) intrapulmonary failure (n = 68), and 2) mediastinal failure (± lung) (n = 25). The two groups were equal in terms of mean age, gender, and GTV (). The distribution of different treatment parameters (chemotherapy and radiotherapy dose) was not statistically significant different in the two groups and evenly distribution in treatment period early versus late was observed. The median time to local recurrence (9.1 months vs. 10.1 months) were of no significant difference in the two groups, where baseline is the date of radiotherapy commencement (). The same was true for survival after relapse (9.4 months vs. 10.1 months).
Figure 2. Kaplan-Meier plot of time to local recurrence, with date of radiotherapy commencement as baseline.
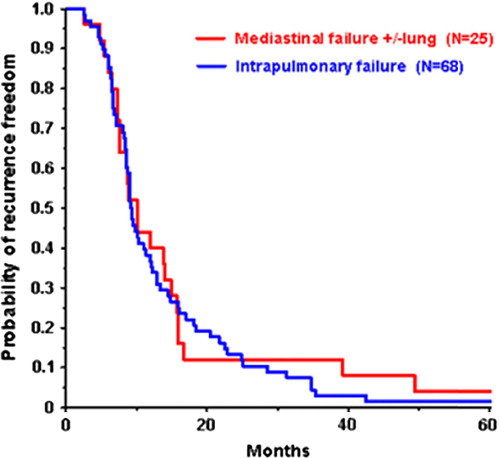
Table II. Ninety-three NSCLC patients with local recurrences only in the divided in “intrapulmonary” failure and “mediastinal” failure (± T-site). Distribution of selected covariates tested in the two groups. Numbers in parenthesis indicates proportions of the group.
When analysing pattern of failure in the population of 331 NSCLC patients treated with definitive radiotherapy, 93 patients (28%) experienced loco-regional failure only as first recurrence. Of these patients, 73% had intrapulmonary failure only, even though the majority (78%) had mediastinal involvement at treatment start.
From the population of 331 patients, the risk of developing intrapulmonary recurrence only was tested in a logistic regression analysis. The only significant risk factor was size of GTV ().
Table III. Logistic regression analyses of the probability of “intrapulmonary”-failure only of the entire population (N = 331 NSCLC patients). The OR is given by the odds for a person being in the group indicated in parenthesis (e.g. male) divided by the odds for a person in the opposite group.
Median overall survival for the study group of 331 patients was 19 months, 20 months for the group of patients with mediastinal relapse, and 19 months for the patient group with intrapulmonary recurrence only.
Discussion
The pattern of loco-regional recurrence after definitive radiotherapy in 331 NSCLC patients was analysed and 93 patients (28%) experienced loco-regional failure only. This result is in line with other studies when analysing at two years follow-up [Citation2,Citation5]. It is, however, remarkable that 73% of the loco-regional recurrences in the current study had intrapulmonary failure only although, 53 of these 68 patients had mediastinal nodal involvement at treatment start. This may indicate that lymph nodes in mediastinum may be cleared at the given dose of 60–66 Gy, and emphasis should focus on improving intrapulmonary control. Furthermore, patients with intrapulmonary failure only are potentially operable when recurrence is diagnosed. Salvage surgery were performed in five patients in whom intrapulmonary relapse was diagnosed. To be candidates for surgery in case of relapse, patients had to have good performance status, good pulmonary function (assessed by spirometer and diffusing capacity for carbon monoxide), and without other major comorbidities.
Evaluation of local control in lung cancer is inherent difficult, and so is comparison between studies. First, there exist different methods of defining loco-regional control (LRC): freedom from local progression (FFLP-LRC), this in contrast to response mandatory loco-regional control (strict-LRC) [Citation17]. FFLP-LRC has better local control rates than strict-LRC at least in the beginning. In the current study local failure were assessed with FFLP-LRC. Second, it is very challenging to distinguish between tumour progression and damaged lung due to radiation. FDG-PET is useful to rule out relapse if the infiltrative changes are without increased FDG uptake. In case of infiltrative changes with increased FDG uptake the investigation is less informative since increased FDG can be observed in both tumour progression and damaged lung due to radiation. The same is true for lymph nodes which can be enlarged and potential FDG positive due to infection, radiation pneumonitis as well as cancer involvement. Third, in previous studies elective node radiation has been used as standard of care. From the current study we conclude that tumour control in mediastinum is not the problem for regional control even though elective nodal irradiation was not performed. This is in line with a randomised trial from Yuan et al. [Citation18] where involved field radiation allowed dose escalation and resulted in better overall response and local control compared to elective nodal irradiation. Similarly results were observed in a newly published retrospective study comparing patients treated with elective nodal irradiation versus involved field irradiation in which elective nodal irradiation did not improve outcome, but gave significantly higher toxicity [Citation19].
The size of GTV was the only significant risk factor for recurrence in lung only in a logistic regression analysis, which might indicate a lack of initial control of large tumours locally. GTV was the combined volume of the tumour and the node, since in practice it was drawn in one volume. We added the T and N stages as covariates in the regression analysis, but both the T and N stages failed to be significant risk factors in the model, indicating that tumour burden is an important prognostic factor for intrapulmonary failure. This is similar to finding in a publication from Bradley et al., who reported GTV was a prognostic factor in terms of local control and survival in NSCLC patients treated with definitive radiotherapy [Citation20].
The current study has some limitations; it is a retrospective study and the population is heterogeneous in terms of treatment (chemotherapy and radiotherapy dose). The radiotherapy planning and technique changed during the decade of study period. In an attempt to take account for these differences a logistic regression with various covariates was performed. During this time period a new staging system for NSCLC was introduced. However, all patients in this study were staged according to AJJC edition 5 [Citation16]. CT scan as part of the regular follow-up schedule was introduced in January 2011, i.e. very late in this study period. Before this change, patients had a CT scan 4–5 weeks after end of radiotherapy as a baseline examination for further follow-up. Another CT scan was performed in case of relapse suspicion, either clinical or radiographic. It can be argued that chest x-ray is not sufficient to evaluate mediastinum and this fact may have influenced the results from this study especially the proportion between mediastinal relapse only and mediastinal as well as intrapulmonary relapse. However, it cannot affect the fact that the majority of the patients had intrapulmonary recurrence only, since a CT scan was performed in case of relapse suspicious. Another issue is that in case of relapse, not all mediastinal lymph nodes were biopsied but only classified as tumour positive due to high FDG uptake or pathologic enlarged. If nodal relapsed had to be cytological proven this might have resulted in further more patients with intrapulmonary relapse only.
The current study has several strengths. It is a large consecutively treated population and no patients are lost in follow-up. In contrast to clinical trials this population includes old patients (the oldest was 87 years) and patients in poor performance status. In this study, 15% were in performance status 2. In this way the population is more representative for everyday practise. Another thing is that all patients have received at least 60 Gy in contrast to clinical trials, where a proportion of the patients cannot go through the treatment due to different reasons, but the analyses are performed as “intention to treat”. The major strengths of this study are, though, that loco-regional failure is separated into intrapulmonary failure and mediastinal failure.
Although data should be interpreted with caution, the data indicate, that patients who are inoperable at diagnosis due to mediastinal involvement may be operable after radiotherapy since lymph nodes in mediastinum have been cleared. Future clinical trials might focus on dose escalation to intrapulmonary tumour since most recurrences are located here and most of the dose-limiting normal tissues (heart, oesophagus etc.) are placed in mediastinum, making partial dose escalation here less attractive. Different methods for escalation have been tested: hyper fractionation [Citation5,Citation21], integrated boost to PET positive area [Citation22], conventional dose escalating [Citation23]. Using inhomogeneous dose distribution is another innovative method to obtain better expected increased tumour control at T site without increasing the risk of acute lung toxicity, similar to the principle behind stereotactic radiotherapy [Citation24]. The principle of inhomogeneous dose escalation in loco-regional advanced NSCLC is manageable, as shown recently in a planning study based on 20 NSCLC patients it is possible to increase tumour control probability with 15 percentage points without increasing toxicity when planning with inhomogeneous dose distribution [Citation15].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work is supported by CIRRO – The Lundbeck Foundation Centre for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics 2. CA Cancer J Clin 2011;61: 69–90.
- Auperin A, Le PC, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small- cell lung cancer. J Clin Oncol 2010;28:2181–90.
- Robinson LA, Ruckdeschel JC, Wagner H, Jr., Stevens CW. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines, 2nd ed. Chest 2007;132(3 Suppl):243S–65S.
- Jett JR, Schild SE, Keith RL, Kesler KA. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines, 2nd ed. Chest 2007;132 (3 Suppl):266S–76S.
- Baumann M, Herrmann T, Koch R, Matthiessen W, Appold S, Wahlers B, et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol 2011;100:76–85.
- Perez CA, Bauer M, Edelstein S, Gillespie BW, Birch R. Impact of tumor control on survival in carcinoma of the lung treated with irradiation. Int J Radiat Oncol Biol Phys 1986;12:539–47.
- Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2012;82:425–34.
- De Ruysscher D, Dehing C, Bentzen SM, Houben R, Dekker A, Wanders R, et al. Can we optimize chemo- radiation and surgery in locally advanced stage III non-small cell lung cancer based on evidence from randomized clinical trials? A hypothesis-generating study. Radiother Oncol 2009; 93:389–95.
- Bentzen SM, Johansen LV, Overgaard J, Thames HD. Clinical radiobiology of squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys 1991;20:1197–206.
- Perez CA, Breaux S, Madoc-Jones H, Camel HM, Purdy J, Sharma S, et al. Correlation between radiation dose and tumor recurrence and complications in carcinoma of the uterine cervix: Stages I and IIA. Int J Radiat Oncol Biol Phys 1979;5:373–82.
- Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833–9.
- Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl): S70–6.
- Nielsen TB, Hansen VN, Westberg J, Hansen O, Brink C. A dual centre study of setup accuracy for thoracic patients based on cone-beam CT data. Radiother Oncol 2012;102: 281–6.
- Sonke JJ, Lebesque J, van HM. Variability of four- dimensional computed tomography patient models. Int J Radiat Oncol Biol Phys 2008;70:590–8.
- Nielsen TB, Hansen O, Schytte T, Brink C. Inhomogeneous dose escalation increases expected local control for NSCLC patients with lymph node involvement without increased mean lung dose. Acta Oncol 2014;53:119–25.
- Sobin LH, Fleming ID. TNM classification of malignant tumors, 5th ed. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80: 1803–4.
- Machtay M, Paulus R, Moughan J, Komaki R, Bradley JE, Choy H, et al. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma. J Thorac Oncol 2012;7:716–22.
- Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol 2007;30:239–44.
- Fernandes AT, Shen J, Finlay J, Mitra N, Evans T, Stevenson J, et al. Elective nodal irradiation (ENI) vs. involved field radiotherapy (IFRT) for locally advanced non-small cell lung cancer (NSCLC): A comparative analysis of toxicities and clinical outcomes. Radiother Oncol 2010;95:178–84.
- Bradley JD, Ieumwananonthachai N, Purdy JA, Wasserman TH, Lockett MA, Graham MV, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2002;52:49–57.
- Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial. CHART Steering Committee. Radiother Oncol 1999;52:137–48.
- van EW, De RD, van der Salm A, Lakeman A, van der Stoep J, Emans D, et al. The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol 2012;104:67–71.
- Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys 2012;82:1042–4.
- Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: An updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552–8.