Abstract
Background. The percentage of people living with a diagnosis of cancer is rising globally. Between 20% and 25% of people treated for cancer experience a consequence of cancer which has an adverse impact on the quality of their life. Gastrointestinal (GI) symptoms are the most common of all consequences of cancer treatment and have the greatest impact on daily activity.
Pathophysiology of long-term bowel damage after pelvic radiotherapy. Long-term damage to the bowel after radiotherapy is mediated by ischaemic changes and fibrosis. Each fraction of radiotherapy causes a series of repetitive injuries to the intestinal tissue resulting in an altered healing process, which affects the integrity of the repair and changes the architecture of the bowel wall.
The nature of GI symptoms that develop. Patient-reported outcome measures show that diarrhoea, urgency, increased bowel frequency, tenesmus and flatulence are the five most prevalent GI symptoms with a moderate or severe impact on patients’ daily lives after treatment with pelvic radiotherapy. Many patients also experience fatigue, urinary problems and have sexual concerns.
Systematic assessment and management. The complex nature of those symptoms warrants systematic assessment and management. The use of a tested algorithm can assist in achieving this. The most common contributing factors to ongoing bowel problems after pelvic radiotherapy are small intestinal bacterial overgrowth, bile acid malabsorption, pancreatic insufficiency, rectal bleeding and its impact on bone health.
The wider context. Symptom burden, socio-psychosocial impact, memory and cognitive function, fatigue, urinary problems and sexual concerns need to be taken into account when thinking about consequences of cancer treatment.
Conclusion. As our understanding of consequences of cancer treatments continues to emerge and encompass a wide variety of specialties, a holistic, multifaceted and multidisciplinary approach is required to manage those consequences long-term.
Setting the scene
The percentage of the population living with a diagnosis of cancer is rising globally [Citation1]. The number of long-term cancer survivors in the UK has tripled over the last three decades. The current increase in numbers of cancer survivors in the UK is 3% per year and in the USA 11% [Citation2]. The increase in cancer prevalence and incidence in Scandinavia is paralleled by the trends seen elsewhere.
The UK National Cancer Survivorship Initiative (NCSI) consequences of treatment work stream estimates that between 20% and 25% of people treated for cancer experience a consequence of cancer which has an adverse impact on the quality of their life [Citation3].
It is difficult to differentiate between therapy modalities as often they are used in combination but gastrointestinal (GI) symptoms are the most common of all consequences of cancer treatment and have the greatest impact on daily activity [Citation4].
After upper GI surgery, 50% of the patients find their GI function has not fully recovered one year post-surgery [Citation2]. Common problems include chronic gut dysmotility, dysphagia, nausea, bloating and reflux but these patients also frequently develop chronic lower GI symptoms such as diarrhoea, urgency and frequency of defaecation. Lower GI surgery also carries a high risk of chronic changes, faecal incontinence and toilet dependency.
Chemotherapy-induced diarrhoea is common but the underlying mechanisms have not been researched extensively even though they are the most frequent cause for delaying further chemotherapy cycles. On treatment with fluoropyrimidines and irinotecan, 50–80% of patients develop acute diarrhoea [Citation5]. Almost all patients undergoing high-dose chemotherapy and stem cell or bone marrow transplantation and 40% of patients treated with standard dose chemotherapy experience mucositis of the whole GI tract [Citation5]. Constipation is also a frequent problem. shows which chemotherapeutic agents are likely to cause diarrhoea or constipation.
Table I. Chemotherapeutic agents likely to cause a change in bowel habit.
The long-term impact on bowel function of treatment with chemotherapy has not been studied. Possible mechanisms include an imbalance in the gut microbiota, malabsorption due to changes in the epithelium, and altered gut motility possibly driven by damage to the enteric nervous system or GI hormone secretion.
About four in 10 people in developed countries who have cancer receive radiotherapy as part of their treatment [Citation6] and in Europe and North America alone it has been estimated that up to 300 000 patients receive pelvic radiotherapy per year.
The optimal management of GI symptoms after cancer treatment is best characterised in patients who have undergone radiotherapy for a pelvic cancer. Research has shown that up to 80% of patients treated with pelvic radiotherapy are left with chronic alteration in GI function and 50% state that these long-term GI symptoms affect their daily activity [Citation7].
Pathophysiology of long-term bowel damage after radiotherapy: The wound that does not heal
The mechanism of how radiotherapy affects the healing process of normal bowel tissue is not fully understood although radiotherapy seems to impact on all phases of the normal healing process. The irradiated bowel tissue resembles a wound that does not heal [Citation8]. Each fraction of radiotherapy causes a series of repetitive injuries to the intestinal tissue resulting in an altered healing process, which affects the integrity of the repair [Citation9].
During treatment, an acute inflammatory response may be associated with diarrhoea but the histological features do not correlate with symptoms. Symptoms typically start two weeks after commencement of treatment and subside after 3–9 months.
Chronic changes in bowel function induced by radiation are no longer mediated by inflammation but by a fibro-atrophic process which is often progressive over time. Fibrosis results in reduced tissue elasticity and in soft tissues may cause symptoms of hardening, distortion and pain. Atrophy contributes to tissue shrinkage and loss of organ function [Citation10]. Changes in intestinal motility and peristalsis may be related, at least in part, to radiation effects on the regulation of the autonomous nerve system and the neuro-peptides involved [Citation11]. The development of telangiectasia is possibly linked to vascular endothelial cell damage [Citation12]. In addition, expression patterns of angiogenic factors and the messenger RNA (mRNA) level in radiation damaged bowel tissue are increased [Citation13]. Scar tissue formation or fibrosis is a normal part of the process of wound healing and usually is composed of the same collagen as it replaces, but after radiotherapy, the fibre composition of the protein is different in comparison to normal tissue. As the architecture of that tissue has changed, the tissue never behaves in the same way as it did before the damage [Citation14].
The nature of GI symptoms after radiotherapy
As a result of the underlying fibro-atrophic and vascular changes in the intestinal wall, and the impact of these changes on normal GI physiological processes, GI function changes and results in symptoms. Twenty-three lower GI symptoms have been identified ().
Table II. Lower gastrointestinal (GI) symptoms.
Several studies have shown that patients frequently present with a constellation of symptoms [Citation15,Citation16] and that those symptoms influence each other; they are interconnected. Therefore a holistic approach to symptoms is paramount in heralding its solution in an effective and efficient fashion.
Recently, the symptom profiles of 110 consecutive patients previously treated with pelvic radiation, and referred to a specialist clinic were analysed. This group included 47 women (43%), median age, 59 (range 37–79 years) and 63 men (57%) median age, 72 years (range 20–83 years) treated for prostate (47%), gynaecological (27%) or anorectal cancers (17%), lymphoma (5%) and other tumours (4%). The median length of time since completing radiotherapy to presenting in clinic was three years and one month (range 0.5–36 years). This is in line with previous research which showed that patients take a long time before seeking professional help to improve their debilitating GI symptoms [Citation17].
Women presented with a median of 12 symptoms (range 2–17) and men with a median of 11 (range 2–16). The median number of symptoms defined by the patients as “frequent” or “severe” was eight symptoms for women (range 0–15) and five symptoms for men (range 0–13).
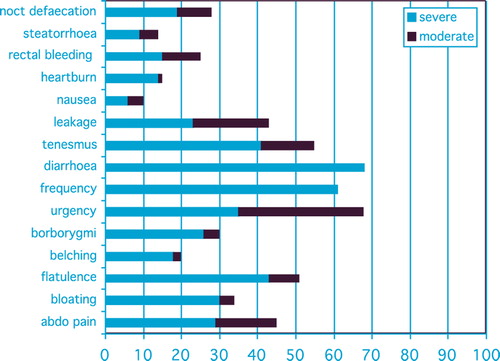
Pelvic symptoms scored by those patients by using patient recorded outcome measures on the Gastro-intestinal Symptom Rating Scale (GSRS) indicate which symptoms have a moderate or severe impact on patients’ daily lives. The Supplementary Questionnaire is available online at http//informahealthcare.com/doi/abs/10.3109/0284186X.2013.873140. The results are shown in .
In addition to the psychological and emotional impact of the high symptom burden experienced by these patients, severe or moderate fatigue (51%), sexual concerns (35%) and urinary problems (34%) were also reported.
Systematic assessment of consequences of cancer treatment
Systematic assessment of the cause for symptoms is rarely carried out [Citation7]. Studies have shown that this is due to the fact that oncology follow-up clinics mainly focus on disease recurrence and referral pathways for symptom management are rarely established. Patients are often embarrassed to discuss GI symptoms such as excessive flatulence or faecal incontinence or believe their symptoms are untreatable; they feel they should not make a “fuss”. Many attribute their symptoms to age or activity or feel that those consequences are acceptable if the trade-off of their cancer treatment is “cure”. Some fear that their symptoms indicate disease recurrence. Sometimes, the invisibility of symptoms to friends and family makes it difficult to discuss them: “you have been cured and you don't look ill”. In addition, patients often try to resolve problems themselves via dietary interventions, anti-diarrhoeal medication or complementary therapies [Citation4,Citation17,Citation18].
Assessment and management of GI symptoms after radiotherapy
The use of Patient Recorded Outcome Measures (PROMS) in our clinic has highlighted the correlation between GI symptoms and quality of life. Patients score their symptoms on a modified GSRS and focus on what the impact of their symptoms is on their daily activity.
Some patients presenting in our specialist clinic have a single clear cause for their symptoms. But often, patients have several identifiable causes contributing to their symptom profile.
Factors unrelated to cancer and its treatment also affect bowel function. Anxiety, stress, underlying GI diseases – often triggered or exacerbated by cancer treatment; such as inflammatory bowel disease (ulcerative colitis, Crohn's disease), new malignancies or malignancies secondary to previous treatment, lactose intolerance, side effects of medication (e.g. diarrhoea with proton pump inhibitors), dietary causes and thyroid function need to be considered first to assess their contribution to current symptomatology.
Apart from excluding several pathological causes that influence bowel function, two major diagnoses are frequently made, alone or in combination, after assessing troublesome GI symptoms after cancer treatment and radiotherapy in particular: small intestinal bacterial overgrowth (38%) and bile acid malabsorption (21%). Exocrine pancreatic insufficiency is less common (5%) after radiotherapy but can easily be excluded. Rectal bleeding is a relatively frequent (12%) occurrence after pelvic radiotherapy due to vascular changes and the development of telangiectasia in the bowel wall, however, it always needs to be adequately assessed endoscopically to exclude other causes [Citation19].
An algorithmic approach to assessing and managing these symptoms has been published elsewhere and its effectiveness has been proven in a landmark clinical trial [Citation20]. In addition, a practical translation into case series demonstrated the benefit of using the algorithm in clinical practice [Citation19]. shows the four key questions that have been recommended by the British Society of Gastroenterologists to aid in identifying patient who need referral to a specialist service [Citation2].
Table III. Four key questions for identifying patients who need referral to a specialist service.
Small intestinal bacterial overgrowth
Small intestinal bacterial overgrowth (SIBO) occurs in 25% of patients during the acute phase of radiotherapy and is a cause of diarrhoea in up to 15% of patients after radiotherapy [Citation7].
SIBO is defined as the presence of excessive bacteria in the small intestine [Citation21]. In the intact intestine, SIBO is prevented by a normally functioning immune system and the actions of gastric acid, pancreatic enzyme activity, small intestinal activity and the ileocaecal valve [Citation22]. Radiotherapy has a direct effect on small bowel motility [Citation23]. Once present, bacterial overgrowth may induce an inflammatory response in the intestinal mucosa exacerbating the symptoms of SIBO [Citation21].
Symptoms of SIBO are often non-specific and very varied and may include bloating, abdominal distension, abdominal pain or discomfort, diarrhoea, flatulence, steatorrhoea and weakness [Citation21,Citation24]. SIBO sometimes contributes to the occurrence of faecal incontinence [Citation25].
Complications of SIBO range from minimal vitamin deficiencies to severe malabsorption, even neuropathy. A common complication is vitamin B12 deficiency due to the use of vitamin B12 by anaerobic bacteria [Citation22]. Blood folate levels are frequently elevated in SIBO due to increased synthesis of folate by small bowel bacteria [Citation26].
The diagnosis of SIBO is difficult and there is no consensus regarding a gold standard test [Citation7,Citation21]. Duodenal aspirate taken at upper GI endoscopy enables micro-organisms to be grown but is invasive, costly and may not always identify organisms which are not easily cultured. Breath testing is the predominant method used to evaluate presence of small bowel bacterial overgrowth [Citation21]. Breath testing is an easy procedure to perform but difficult to interpret. A glucose hydrogen breath test has been reported to have a sensitivity of 39–93% and a specificity of 75–100% at detecting small bowel bacterial overgrowth [Citation25].
Ideally, antibiotic therapy is based on bacterial culture and sensitivity. Often, antibiotics are used empirically to treat SIBO [Citation21]. The presence of several bacterial species with potentially different antibiotic sensitivities requires the administration of broad-spectrum antibiotics [Citation24].
Symptoms can recur any time after antibiotics are stopped because the underlying cause for bacterial overgrowth cannot usually be addressed. If symptoms return, repeat treatment with antibiotics for a few days every month or continually at the lowest effective dose may be helpful in managing symptoms long-term [Citation2].
Treatment decisions should be individualised and take into account the risks of long-term antibiotic therapy such as Clostridium difficile infection, cumulative, potentially irreversible neuropathy after use with metronidazole, intolerance, bacterial resistance, idiopathic side effects and cost [Citation24].
Bile acid malabsorption
In healthy individuals, over 95% of bile acids are reabsorbed in the terminal ileum and the bile acid pool size is maintained though positive and negative feedback mechanisms [Citation27].
Bile acid malabsorption (BAM) is defined as a defect in the enterohepatic circulation of bile acids in the terminal ileum. This causes symptoms related to diarrhoea due to excess levels of unabsorbed bile acids in the colon.
Two major types of BAM have been identified: ileal dysfunction whereby the ability to absorb bile acids in the terminal ileum is impaired and secondly, hepatic overproduction which overwhelms terminal ileal absorption capacity [Citation28]. More rapid small bowel transit results in a reduced time in the ileum to allow absorption [Citation29]. Increased hepatic bile acid production, saturation of the uptake mechanism, altered enterohepatic cycling and reduced storage capacity can all account for bile acid spilling over in the colon resulting in watery diarrhoea [Citation28,Citation30].
The usually effective enterohepatic circulation of bile salts is most obviously deranged in ileal disease. Following ileal resection, typically for Crohn's disease or caecal cancer, bile acids are not absorbed efficiently, resulting in clear cut BAM. Inflammation without resection can also affect bile acid absorption. Many other intestinal conditions, such as any form of upper GI surgery, SIBO, pancreatitis or pancreatic insufficiency can interfere with the normal physiology of bile acid reabsorption [Citation28].
Whilst BAM is not life threatening, the symptom burden can severely impair quality of life. Patients with mild to moderate bile acid malabsorption present with erratic loose stool (type 5–7 Bristol Stool Chart) – they may be relatively constipated between episodes – while those with severe malabsorption may also have steatorhoea [Citation29]. Other symptoms of BAM include, urgency, bowel frequency, unpredictability of bowel motions, abdominal colic, and flatulence. Consequences of abnormal lipid digestion lead to malnutrition with malabsorption of fat- soluble vitamins (A-D-E-K) and trace elements and require supplementation.
Untreated BAM may increase the risk of gallstone and renal stone formation. There is often associated vitamin B12 deficiency with fatigue and dyspnoea [Citation28].
A definitive diagnosis of BAM is made by performing a 23- [75Se] Selena-25-homocholic acid taurocholoate (SeHCAT) scan. SeHCAT is a synthetic bile acid which passes through the enterohepatic circulation. Its levels of retention can be measured by calculating the whole body retention after seven days, using an uncollimated gamma camera.
The SeHCAT scan result indicates the severity of BAM (). In contrast to what was previously thought idiopathic bile acid malabsorption (I-BAM) is not as rare as previously estimated (prevalence 1%), and is an important cause of diarrhoea- predominant IBS type symptoms [Citation30]. The prevalence of BAM after pelvic radiotherapy has varied at 1–85% between studies. Recent data suggest that the development of BAM happens even after fairly low dose radiation exposure of the terminal ileum [Citation31].
Table IV. Severity scores of bile acid malabsorption.
Optimal treatment of BAM is inadequately defined. Our approach to this common, debilitating problem is to offer specialist dietary assessment and advice about a low fat diet and vitamin supplementation for mild and moderate BAM in the first instance and the use of bile acid sequestrants in combination with dietary changes for severe BAM. Two different types of bile acid sequestrants are available. There are two similar resins, colestyramine (Questran) and colestipol (Colestid). About one in four people do not tolerate the taste or they make diarrhoea worse or cause intolerable nausea, heartburn, wind or bloating. If steatorrhoea is present, these agents usually make it worse. A better tolerated alternative is the tablet Colesevelam in Europe. Colesevelam is available as 625 mg film-coated tablets and the dose should be increased slowly over six days. Most people take between two and seven tablets a day in two or three doses, with food.
Lifestyle changes regarding diet are based on reducing fat intake. In the southern half of the UK, the average adult eats 120 g fat per day. The recommended amount for men is 95 g and for women 70 g per day. To manage BAM, fat intake needs to be reduced to 20% of the daily calorie intake through fat (40–60 g/day) and patients need considerable help to achieve this. The use of a seven-day food diary enables tailored dietary advice based on the individual patient's habits and likes.
Treatment requires a specialist multidisciplinary approach with extensive patient information, teaching and the use of motivational communication techniques.
Exocrine pancreatic insufficiency
The pancreas is thought to be a relatively radio- resistant organ, however, pancreatic insufficiency after pelvic radiotherapy and para-aortic lymph node irradiation does occur [Citation32,Citation33].
Exocrine pancreatic insufficiency (EPI) is defined as the inadequate production and secretion of pancreatic enzymes. Patients present with symptoms of fat malabsorption when < 10% of their exocrine pancreatic function remains. At lesser levels of insufficiency, they may have bloating, discomfort, weight loss or diarrhoea. Protein and starch digestion are usually maintained [Citation34,Citation35]. EPI can cause or exacerbate gut motility disorders due to alterations in the neurohormonal regulation of intestinal motility [Citation35]. Diagnostic options include indirect measures (faecal elastase). Human faecal elastase 1 (E1) with a cut-off value of < 200 μg elastase 1 per gram of stool is diagnostic except when significant small bowel bacterial overgrowth is present when stool levels of faecal elastase can be very low [Citation36]. The (secretin-cerulin or secretin-pancreozymin) tests are direct measures but are time consuming, expensive and only available in specialist centres [Citation34]. Quantification of faecal fat is difficult and increasingly unavailable.
Treatment of exocrine pancreatic insufficiency involves pancreatic enzyme replacement therapy with dietary modification sometimes also required. As with BAM, consequences of abnormal lipid digestion lead to malnutrition with malabsorption of fat- soluble vitamins (A-D-E-K) and trace elements. Nutritional assessment, patient counselling and support in making dietary and life style changes are integral to its management.
Rectal bleeding
Rectal bleeding has been reported to occur in up to 53% of patients who previously received pelvic radiotherapy with implications for quality of life requiring intervention in fewer than 6% [Citation7,Citation37]. The onset of rectal bleeding is usually at 3–12 months after radiotherapy for prostate cancer and worsens symptomatically over several years before gradually improving spontaneously [Citation38]. There are few data on the natural history of rectal bleeding after treatment for gynaecological cancers.
Microscopic changes of damage to the vascular endothelial cells after radiotherapy may progress to destruction of those epithelial cells in the rectal mucosa and can result in mucosal ulcers and neovascularisation [Citation39]. The inflammatory process stimulates regenerative processes which result either in mucosal repair or worsen the inflammation with ulceration and progressive fibrosis [Citation7].
Due to vascular telangiectasia or non-healing ulceration, severe recurrent haemorrhage can occur [Citation38]. The dose of radiotherapy delivered to the anterior rectal wall is closely related to the risk of bleeding from telangiectasia [Citation40]. Progressive chronic ischaemia caused by vascular damage also predisposes to other long-term complications such as ulceration, strictures or incontinence [Citation41].
Patients with rectal bleeding should be managed like any other high-risk GI bleed and patients should be offered at least flexible sigmoidoscopy because of the high prevalence of unexpected pathology and to exclude other causes of the bleeding [Citation7,Citation37]. Radiotherapy is not the cause of rectal bleeding in 25–60% of the patients presenting with this symptom [Citation7].
Medical treatments for radiation-induced rectal bleeding include sucralphate enemas, pentosan polysulphate, metronidazole, vitamin A, thalidomide and hyperbaric oxygen therapy [Citation37,Citation38,Citation42]. Evidence for the benefits of endoscopic therapy is entirely based on published clinical series. These include thermal coagulation therapy including YAG laser, Argon laser, bipolar electrocoagulation and heater probe treatment.
All thermal therapies and particularly argon plasma coagulation (APC) are used frequently but carry a high risk of causing damage to the bowel wall.
Endoscopic intervention may be sufficient for bleeding from discrete sites, but interventional radiology with embolisation or surgery may be required very rarely in extensive mucosal change [Citation37].
Another option includes the use of endoscopic application of formalin. In medicine, a solution of formaldehyde, a simple aldehyde gas, in water (formalin) is mainly used as a disinfectant, as a preservative for biological tissue samples or to treat warts.
The use of formalin for radiation-induced bleeding, originates from its use for the treatment of haematuria from radiation cystitis [Citation43]. Rubenstein and his colleagues applied this method in 1986 to treat rectal bleeding induced by radiation [Citation44]. Formalin chemically cauterises by hydrolysing protein and superficially coagulating the tissue. Endoscopic formalin application continues to be used frequently but at different concentrations (1–10%), contact time, volumes and methods of instillation, including the use of formalin-soaked gauze [Citation42].
In general, the procedure seems to be effective, safe, well tolerated by the patient, inexpensive, technically simple and can be done in an outpatient setting [Citation42].
Vitamin D deficiency, bone health and muscle cramps
Cancer treatments such as hormonal treatment, chemotherapy (especially methotrexate and ifosfamide) weaken bone structure directly and surgery induced changes in hormone levels increase the risk of bone loss and osteoporosis. Rheumatoid arthritis and coeliac's disease also predispose to osteoporosis. Age (> 50), gender (women), physical activity, diet and calcium intake, a family history of osteoporosis, previous bone fractures over the age of 50, weight (BMI < 19), smoking habits and other medications such as anti-convulsants, heparin, proton pump inhibitors and corticosteroids affect bone health [Citation45,Citation46]. When a patient has been diagnosed with bile acid malabsorption or pancreatic insufficiency, fat soluble vitamins are less well absorbed and vitamin D deficiency is common [Citation28].
Vitamin D levels should be checked yearly and appropriate management should be instituted together with life style advice.
In addition to vitamin D deficiency, malabsorption of the other fat soluble vitamins (A, E and K) or reduced magnesium levels commonly occur. Patients complaining of muscle cramp often find symptom relief after appropriate supplementation [Citation47].
The wider context
Symptom burden and the socio-psychological impact
GI consequences of cancer treatment are the most troublesome and have a high symptom burden. The impact on emotional and psychological wellbeing is well established [Citation4,Citation17].
Body image problems due to weight loss or weight gain, especially due to changes in hormone levels is often reported both by men and women.
Problems making plans due to having an erratic and unpredictable bowel function can result in severe disruption of social activity and the ability to work. This also has financial and socioeconomic implications as many patients describe having to buy incontinence pads or new clothes when having had an episode of incontinence in public or have increased laundry costs. In addition, the psychological remnant of the experience often leaves patients a very limited choice to interact socially.
After treatment for cancer, many patients face problems with memory and cognitive function [Citation48]. A referral to a memory clinic early on can help patients significantly.
Fatigue
The etiological pathopsychophysiology underlying cancer-related fatigue is multifactorial and not well delineated [Citation49]. Mechanisms may include abnormal accumulation of muscle metabolites, dysregulation of the homeostatic status of cytokines, irregularities in neuromuscular function, abnormal gene expression, inadequate ATP synthesis, serotonin dysregulation with increased levels and abnormal vagal afferent nerve activation. A recent study showed that fatigue after cancer is central and not peripheral. Patients did not have lower muscle endurance [Citation50]. Alterations in circadian function have been demonstrated in patients with cancer. These include changes in endocrine rhythms (cortisol, melatonin, and prolactin secretion), metabolic processes (temperature and circulating protein levels), the immune system (levels of circulating leukocytes and neutrophils), and rest–activity patterns [Citation51]. Evidently, disrupted sleep and insomnia contribute to fatigue [Citation52].
Identification and treatment of associated comorbidities, such as anaemia, thyroid dysfunction, pain, insomnia, malnutrition, and other comorbid conditions can make a vast difference in levels of fatigue for cancer survivors.
An array of psychosocial mechanisms, including self-efficacy, causal attributions, emotional distress, expectancy, coping, and social support are linked to fatigue and low energy levels [Citation49].
Practically, the symptom burden of chronic altered bowel function with either diarrhoea or constipation can reduce physical activity in a patient who is housebound due to the severity or frequency of their symptoms. Women with substantial diarrhoea after pelvic radiotherapy report more fatigue and 22% experience limitations in performing work and household tasks [Citation53]. The intensity of fatigue is also positively correlated with the severity of diarrhoea [Citation54].
As well as assessing the underlying contributing factors, there is substantial evidence suggesting that the physical activity recommendations developed by the Department of Health in the UK are sufficient for cancer survivors [Citation55]: 30 minutes of moderate intensity physical activity on five or more days of the week. The most recent expert advice emphasises that even a modest amount of exercise like brief walks is beneficial, and improves core fitness [Citation55].
Urinary problems
Urinary and bowel symptoms often go hand in hand and influence each other. This includes increased urinary frequency, urinary incontinence (urge, stress or mixed) and urinary leakage, nocturia, incomplete emptying, post-micturition dribble, increased frequency of urinary tract infections, pain on micturition or haematuria due to telangiectasia formation in the lining of the bladder wall.
Management of urinary incontinence often includes the use of anti-muscarinic medication (e.g. oxybutinin) to treat an overactive bladder which impacts on bowel function via its action on the nervus vagus and causes constipation [Citation56].
A recent study linked urinary urge incontinence to having more difficulty to postpone defaecation, resulting in faecal incontinence and showed that urinary stress incontinence is positively correlated to experiencing faecal leakage when passing wind [Citation57].
In general, urinary problems, especially incontinence, have been recognised as having a great impact on quality of life, work productivity, sexuality and emotional well-being [Citation58]. It is therefore essential that this link is acknowledged in people who have been treated for cancer.
Research into the importance of pelvic floor exercises to improve both bowel and bladder control has been mainly focused on women but the same principles can also be applied to men [Citation17].
Sexual concerns
The psychosexual impact of cancer and cancer treatment is influenced not only by the physical changes that happen due to treatment but also by the emotional and psychological effects on the person [Citation59].
Physical problems include vaginal dryness, dyspareunia, erectile dysfunction, ejaculation difficulties, loss of libido, reduced orgasmic response, changes to sexual organs, hot flushes and fertility problems [Citation59–61].
Hormone treatment used in patients with breast cancer and prostate cancer patients result in altered hormone levels and diminished sexual desire [Citation61]. Nerve damage after surgical treatment for rectal cancer can result in problems in sexual functioning. This is also worse in patients with a stoma. Preoperative radiotherapy has an additional effect [Citation60].
The link between urinary problems, bowel problems – especially incontinence – and sexual concerns has been acknowledged and should feature in the general assessment of consequences of cancer treatments [Citation62].
Consideration of consequences of cancer treatments: Conclusion
As our understanding of consequences of cancer treatments continues to emerge and encompass a wide variety of specialties, a multifaceted approach is required. The implications are widespread and increased awareness is vital to achieve consideration of those consequences earlier in the cancer patient pathway. More so is the need to establish and develop referral pathways and appropriate follow-up for the growing numbers of cancer survivors experiencing these issues. A systematic and holistic approach is paramount in assessing and managing these patients, especially in relation to the wider context of GI consequences of cancer treatment.
Supplementary Questionnaire
Download PDF (130.8 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ann Muls is funded by Macmillan Cancer Support for a period of 3 years. This paper has been written to reflect the presentation of the Acta Oncologica Lecture in Linköping, Sweden, on March 21st, 2013.
References
- Maher J, McConnell H. New pathways of care for cancer survivors: Adding the numbers. Br J Can 2011;105:S5–10.
- Andreyev HJN, Davidson S, Gillespie C, Allum W, Swarbrick E. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 2012;61:179–92.
- Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C, et al. Patients’ supportive care needs beyond the end of treatment: A prospective and longitudinal survey. J Clin Oncol 2009;27:6172–9.
- Abayomi J, Kirwan J, Hackett A. The prevalence of chronic radiation enteritis following radiotherapy for cervical or endometrial cancer and its impact on quality of life. Eur J Oncol Nurs 2009;13:262–7.
- Gibson R, Keefe D. Cancer chemotherapy-induced diarrhoea and constipation: Mechanisms of damage and prevention strategies, J Support Care Cancer 2006;14: 890–900.
- Cancer Research UK 2012. Incidence of cancer diagnosis in the UK. [cited 2013 Feb 09]. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/prevalence/prevalence-uk
- Andreyev HJN. Gastrointestinal symptoms after pelvic radiotherapy: A new understanding to improve management of symptomatic patients. Lancet Oncol 2007;8:1007–17.
- Martin M, Lefaix JL, Delanian S. TGF-ß1 and radiation fibrosis: A master switch and a specific therapeutic target?Int J Radiat Oncol Biol Phys 2000;47:277–90.
- Denham J, Hauer-Jensen M. The radiotherapeutic injury: A complex ‘wound’. Radiother Oncol 2002;63:129–45.
- Westbury CB, Yarnold JR. Radiation fibrosis – current clinical and therapeutic perspectives. Clin Oncol 2012; 24:1–16.
- Trott K, Doerr W, Facoetti A, Hopewell J, Langendijk J, van Luijk P, et al. Biological mechanisms of normal tissue damage: Importance for the design of NTCP models. Radiother Oncol 2012;105:79–85.
- Giotopoulos G, Symonds RP, Foweraker K, Griffin M, Peut I, Osman A, et al. The late radiotherapy normal tissue injury phenotypes of telangiectasia, fibrosis and atrophy in breast cancer patients have distinct genotype-dependent causes. Br J Cancer 2007;96:1001–7.
- Takeuchi H, Kimura T, Okamoto K, Aoyagi E, Miyamoto H, Kaji M, et al. A mechanism for abnormal angiogenesis in human radiation proctitis: Analysis of expression profile for angiogenic factors. J Gastroenterol 2012;47:56–64.
- Gurtner G, Werner S, Barrandon Y, Longaker M. Wound repair and regeneration. Nature 2008;453:314–21.
- Benton B, Norton C, Lindsay J, Dolan S, Andreyev HJN. Can nurses manage gastrointestinal symptoms arising from pelvic radiation disease?Clin Oncol 2011;23:538–51.
- Capp A, Inostroza-Ponta M, Bill D, Moscato P, Lai C, Christie D, et al. Is there more than one proctitis syndrome? A revisitation using data from the TROG 96.01 trial. Radiother Oncol 2009;90:400–7.
- Gillespie C, Goode C, Hackett C, Andreyev HJN. The clinical needs of patients with chronic gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2007; 26:555–63.
- Faithfull S. ‘Just grin and bear it and hope that it will go away’: Coping with urinary symptoms from pelvic radiotherapy. Eur J Cancer Care Engl 1995;4:158–65.
- Muls A, Watson L, Shaw C, Andreyev HJN. Managing gastrointestinal symptoms after cancer treatment. A practical approach for gastroenterologists. Frontline Gastroenterol 2013;4:57–68.
- Andreyev HJN, Benton B, Lalji A, Norton C, Mohammed K, Gage H, et al. Algorithm-based management of patients with gastrointestinal symptoms in patients after pelvic radiation treatment (ORBIT): A randomised controlled trial. Lancet 2013;382:2084–92.
- Dukowicz A, Lacy B, Levine G. Small bowel bacterial overgrowth: A comprehensive review. Gastroenterol Hepatol 2007;3:112–22.
- Quigley E, Quera R. Small intestinal bacterial overgrowth: Roles of antibiotics, prebiotics and probiotics. Gastroenterology 2006;130:S78–90.
- Yeoh E, Horowitz M, Russo A, Muecke T, Robb T, Maddox A, et al. Effect of pelvic radiation on gastrointestinal function. Am J Med 1993;95:397–406.
- Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, et al. Small intestinal bacterial overgrowth: Diagnosis and treatment. Dig Dis 2007;25:237–40.
- Wedlake L, Thomas K, McCough C, Andreyev HJN. Small bowel bacterial overgrowth and lactose intolerance during radical pelvic radiotherapy: An observational study. Eur J Can 2008;44:2212–7.
- Zaidel O, Lin H. Uninvited guests: The impact of small intestinal bacterial overgrowth on nutritional status. Prac Gastroenterol 2003;7:27–34.
- Johnston I, Nolan J, Pattni SS, Walters JR. New insights into bile acid malabsorption. Curr Gastroenterol Rep 2011;13: 418–25.
- Walters J, Pattni S. Managing bile acid malabsorption. Therap Adv Gastroenterol 2010;3:349–57.
- Pattni S, Walters J. Recent advances in the understanding of bile acid malabsorption. Br Med Bull 2009;92:1–15.
- Wedlake L, Hern R, Russell D, Thomas K, Walters J, Andreyev HJN. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30: 707–17.
- Harris V, Benton B, Sohaib A, Dearnaley D, Andreyev HJN. Bile acid malabsorption after pelvic and prostate intensity modulated radiation therapy: An uncommon but treatable condition. Int J Radiat Oncol Biol Phys 2012;84:e601–6.
- Kingham J, Barrett A. Pancreatic insufficiency following abdominal irradiation. Postgrad Med J 1980;56:804–5.
- Dookeran K, Thompson M, Allum W. Pancreatic insufficiency secondary to abdominal radiotherapy. Eur J Surg Oncol 1993;19:95–6.
- Chowdhury R, Forsmark C. Review article: Pancreatic function testing. Aliment Pharmacol Ther 2003;17:733–50.
- Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: Present and future. Clin Exp Gastroenterol 2011;4:55–73.
- Löser C, Möllgaard A, Fölsch U. Faecalelastase1: A novel, highly sensitive, and specific tubeless pancreatic function test. Gut 1996;39:580–6.
- Andreyev HJN, Wotherspoon A, Denham J, Hauer-Jensen M. “Pelvic radiation disease”: New understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol 2011;46:389–97.
- Denton A, Andreyev HJN, Forbes A, Maher EJ. Systematic review for non-surgical interventions for the management of late radiation proctitis. Br J Can 2002;87:134–43.
- Cullen S, Frenz M, Mee A. Treatment of haemorrhagic radiation-induced proctopathy using small volume topical formalin instillation. Aliment Pharmacol Ther 2006;23: 1575–9.
- Sanguineti G, Franzone P, Marcenaro M, Foppiano F, Vitale V. Sucralfate versus mesalazine versus hydrocortisone in the prevention of acute radiation proctitis during conformal radiotherapy for prostate carcinoma. A randomized study. Strahlenther Onkol 2003;179:464–70.
- Konishi T, Watanabe T, Kitayama J, Shibahara J, Nagawa H. Endoscopic and histopathologic findings after formalin application for hemorrhage caused by chronic radiation- induced proctitis. Gastrointest Endosc 2005;61:161–4.
- Raman R. Two percent formalin retention enemas for haemorrhagic radiation proctitis: A preliminary report. Dis Col Rectum 2007;50:1–8.
- Shrom S, Donaldson M, Duckett J, Wein A. Formalin treatment for intractable hemorrhagic cystitis: A review of the literature with 16 additional cases. Cancer 1976;38:1785.
- Rubinstein E, Ibsen T, Rasmussen R, Reimer E, Sorensen B. Formalin treatment of radiation-induced hemorrhagic proctitis. Am J Gastroenterol 1986;81:44–5.
- Pitts C, Kearns A. Update on medications with adverse skeletal effects. Mayo Clin Proc 2011;86:338–43.
- Muir J, Andrew M, Hirsh J, Weitz J, Young E, Deschamps P, et al. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo. Blood 1996;88: 1314–20.
- Khoshknabi D. Muscle spasms. In: Walsh D, Caraceni AT, Fainsinger R, Foley K, Glare P, Goh C, et al., editors. Palliative Medicine, 1st ed. Philadelphia, Pa: Saunders Elsevier; 2008. Chapter 168.
- Phillips K, Jim H, Small B, Laronga C, Andrykowski M, Jacobsen P. Cognitive functioning after cancer treatment. A 3-year longitudinal comparison of breast cancer survivors treated with chemotherapy or radiation and noncancer controls. Cancer 2012;118:1925–32.
- Mustian K, Morrow G, Carroll J, Figueroa-Moseley C, Jean-Pierre P, Williams G. Integrative nonpharmacologic behavioural interventions for the management of cancer related fatigue. Oncologist 2007;12(Suppl 1):52–67.
- Kisiel-Sajewicz K, Davis M, Siemionow V, Seyidova-Khoshknabi D, Wyant A, Walsh D, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44:351–61.
- Ryan J, Carroll J, Ryan E, Mustian K, Fiscella K, Morrow G. Mechanisms of cancer-related fatigue. Oncologist 2007;12(Suppl 1):22–34.
- Roscoe J, Kaufman M, Matteson-Rusby S, Palesh O, Ryan J, Kohli S, et al. Cancer-related fatigue and sleep disorders. Oncologist 2007;12(Suppl 1):35–42.
- Bye A, Tropé C, Håvard Loge J, Hjermstad M, Kaasa S. Health-related quality of life and occurrence of intestinal side effects after pelvic radiotherapy: Evaluation of long-term effects of diagnosis and treatment. Acta Oncol 2000;39:173–80.
- Ahlberg K, Ekman T, Gaston-Johansson F. The experience of fatigue, other symptoms and global quality of life during radiotherapy for uterine cancer. Int J Nurs Stud 2005;42: 377–86.
- Davies N, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: A review of the literature. Br J Cancer 2011;105(Suppl 1):S52–73.
- Abrams P, Andersson K, Birder L, Brubaker L, Cardozo L, Chapple C, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurol Urodyn 2010;29:213–40.
- Wyndaele M, de Winter B, Pelckmans P, Wyndaele JJ. Lower bowel function in urinary incontinent women, urinary continent women and in controls. Neurol Urodyn 2011;30: 138–43.
- Coyne K, Sexton C, Irwin D, Kopp Z, Kelleher C, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: Results from the EPIC study. Br J Urol Int 2008;101:1388–95.
- Sundquist K. Sexuality and body image after cancer. Aust Fam Physician 2003;32:19–22.
- Lange M, Marijnen C, Maas C, Putter H, Rutten H, Stiggelbout A, et al. Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer 2009;45:1578–88.
- Sadovsky R, Basson R, Krychman M, Morales AM, Schover L, Wang R, et al. Cancer and sexual problems. J Sex Med 2010;7:349–73.
- Vistad I, Cvancarova M, Fossa S, Kristensen G. Postradiotherapy morbidity in long-term survivors after locally advanced cervical cancer: How well do physicians’ assessments agree with those of their patients?. Int J Radiat Oncol Biol Phys 2008;71:1335–42.