Abstract
Background. Chemoradiotherapy (CRT) for squamous cell carcinoma of the anus (SCCA) may cause significant toxicity, and concerns exist about its tolerability in the elderly. The authors compared tolerability and outcomes across the age groups following CRT for SCCA.
Methods. Single-institution retrospective analysis of patients with localized SCCA treated with CRT. CRT was standardized at 50.4–54 Gy, with concurrent infusional 5-fluorouracil and mitomycin C. Patients were arbitrarily categorized into three groups: Group 1 – age < 50 years; Group 2 – age ≥ 50 and < 70 years; and Group 3 – age ≥ 70 years.
Results. Of 284 patients identified, 278 were evaluable. The number of patients in each age group was: Group 1 – 51; Group 2 – 140; and Group 3 – 93. Baseline and treatment characteristics, tumor stage, rates of overall acute toxicity, need for unplanned treatment breaks and chemotherapy delivery were largely similar across the age groups. However, nine patients in Group 3 did not complete CRT, compared with five and none in Groups 1 and 2, respectively (p = 0.006). In addition, five patients in Group 3 had diarrhea requiring treatment break, compared with none in the other two groups (p = 0.004). At a median follow-up 5.3 years, there was no significant difference in overall survival (p = 0.11), disease-free survival (p = 0.22) or local-recurrence free survival (p = 0.34), across the three age groups.
Conclusions. CRT is safe and tolerable in the elderly age group, and provides equivalent disease control rates compared with the younger age group. Age alone should therefore not preclude aggressive curative treatment.
Concurrent chemoradiotherapy (CRT) has the potential to cure a significant proportion of patients with non-metastatic anal cancer [Citation1]. However, curative treatment comes at the expense of significant acute toxicity, even in the young and fit patient, not only from the frequently large radiation volumes for nodal prophylaxis, but also the added toxicities of concurrent chemotherapy.
There are concerns about the ability of the elderly age group (≥ 70 years) to tolerate aggressive treatment, many of whom have significant comorbidity, frailty or poor performance status. These factors have implications with regards to chemotherapy toxicity and tolerability, including prolonged myelosuppression with mitomycin C (MMC), renal toxicity and prehydration requirements of cisplatin.
Various treatment modifications have been employed in an attempt to improve treatment tolerability for elderly patients, including reduced radiation dose, omitting prophylactic nodal irradiation, chemotherapy dose reduction, single chemotherapy agent with 5-fluorouracil (5-FU) alone, as well as implementing a planned treatment break. These measures may potentially compromise tumor control, but are often considered reasonable options with the presumption that this group of patients may have a limited lifespan.
There is a lack of studies evaluating the treatment of elderly patients with anal cancer. The direct extrapolation of benefit of curative CRT for elderly patients is hampered not only by the fact that some studies excluded the elderly subgroup [Citation2,Citation3], but also that the median age of patients in the large randomized trials ranged from 55 to 65 years, with an under-representation of patients aged ≥ 70 years [Citation1,Citation2]. The burden of anal cancer in this population is not insubstantial, with Australian Cancer Registry statistics [Citation4] showing over 40% of new anal cancer diagnoses occurring in patients over the age of 70 years, with 16% occurring above 80 years.
This study describes our experience treating patients with anal cancer across all age groups using a uniform treatment policy for the past 25 years. The aims are to compare the toxicity, outcomes and overall survival of patients in different age groups treated with curative intent CRT.
Material and methods
This is a single-institution retrospective review. Consecutive patients with non-metastatic squamous cell carcinoma of the anus treated with curative intent CRT between February 1983 and February 2008 were analyzed. Patients were staged by physical examination, examination under anesthesia/proctoscopy, chest radiograph or computed tomography (CT) of the chest, CT of abdomen and pelvis, and in the latter part of the study period, endorectal ultrasound, pelvic magnetic resonance imaging (MRI), and positron emission tomography (PET). Suspected groin node involvement was confirmed with fine needle aspiration or excisional biopsy. Details of data collection and treatment have been described elsewhere [Citation5]. A protocol and an analysis plan were established before commencement of this study. The study was approved by the institutional ethics board.
For the purposes of this study, patients were arbitrarily categorized into three age groups, which were pre-determined prior to data analysis: Group 1 – age < 50 years; Group 2 – age ≥ 50 and < 70 years; and Group 3 – age ≥ 70 years.
All patients were managed by the gastrointestinal team of the cancer center. The unit policy remained consistent during the study period. Patients were treated with CRT to a total dose of 50.4–54 Gy in 1.8 Gy per fraction with concurrent infusional 5-FU and MMC.
Radiotherapy (RT) was generally delivered in a three-phase technique, with the first phase encompassing the primary tumor, lower mesorectal, presacral, lower internal and external iliac, and inguinal lymph nodes. The standard technique utilized wide antero-posterior/postero-anterior photon beams to 36 Gy in 20 fractions, with the superior field border 1 cm above the inferior sacroiliac joints or 5 cm proximal to the primary tumor, whichever was more proximal. The second phase then treated the primary tumor, distal internal iliac and mesorectal nodal regions, usually with a three-field arrangement of posterior and parallel-opposed lateral photon beams, a further 9 Gy in 5 fractions, to 45 Gy in 25 fractions. The third phase boosted the primary tumor to 5.4–9 Gy in 3–5 fractions (bringing the total dose of 50.4–54 Gy in 28–30 fractions). Patients with T1 N0 tumors did not routinely have elective inguinal radiation, and were treated with a technique akin to the second phase described above (to 45 Gy in 25 fractions with a three-field arrangement), followed by a boost to the primary tumor similar to the third phase above.
Patients were reviewed weekly during the course of CRT, fortnightly after completion of CRT until resolution of acute toxicity, three monthly for three years, six monthly for another two years and then yearly. Routine reviews consisted of history and clinical examination including digital rectal examination. Biopsies were performed if there was clinical suspicion of persistent or recurrent disease.
Data on the type of acute toxicity which resulted in interruption of treatment was collected, and data from subsequent visits about survival and recurrence status recorded. Local recurrence was defined as persistence (at least 12 weeks following CRT) or recurrence of disease following a complete response.
The differences in baseline and treatment characteristics between the ordered three age groups were assessed by the p-value corresponding to the Score test in ordered logistic regression (with ordered age group as the response) or the Type I sum of squares F-value in ANOVA, as appropriate. Median follow-up was assessed by the reverse Kaplan-Meier method. Local recurrence free, distant recurrence free and overall survival from the date of CRT completion were assessed with Kaplan-Meier curves, with the Cox proportional hazards regression unadjusted and adjusted for T stage and sex used to compare survival between the three age groups.
In addition, post-hoc sensitivity analyses were performed using varying age cut-offs (> 75 years and > 80 years) for Group 3. These were done following the initial analysis to determine whether the initial conclusions were likely to change when using varying age parameters.
Results
There were 284 patients identified with localized squamous cell carcinoma of the anal canal treated with definitive CRT at the cancer center between 1983 and 2008. There were five patients without any response or follow-up data following radiotherapy or the post-treatment PET scan, and one patient with response data but no further follow-up, thus leaving 278 patients for the outcome analyses. When grouped by age at diagnosis, 18% of patients (n = 51) were aged < 50 years, 50% of patients (n = 140) aged 50–69 years, and 33% (n = 93) of patients were aged 70 years or older. There were 25 patients aged 80 years or older.
Baseline characteristics
Baseline characteristics of all patients (n = 284) are outlined in , and were similar across patients of different age groups with regards to sex, primary tumor site, diameter, differentiation, T stage and N stage. There was a suggestion that younger patients were more likely to have higher AJCC staging (p = 0.05), yet this trend was not observed when the sensitivity analyses were done (p = 0.24) (Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513).
Table I. Baseline patient characteristics.
Treatment characteristics
Treatment characteristics are listed in . Ninety- five percent of all patients completed CRT. Most patients {63% [95% confidence interval (CI) 57–69%]} completed their radiotherapy with no break. However, nine patients aged > 70 years did not complete CRT, compared with five in the 50–70-year-old age group, and none in the younger < 50 years age group; this difference was statistically significant (p = 0.006). Only two of 25 patients aged 80 years or above did not complete CRT. Otherwise, treatment details were largely similar across the different age groups with respect to the need for unplanned treatment breaks and the length of treatment breaks. There were no significant differences in terms of chemotherapy regimens or dose intensity among the age groups.
Table II. Treatment characteristics.
Sensitivity analyses confirmed a significant association of age with completion of RT even when a cut-off of > 75 years was used (Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513).
Acute toxicity
Thirty-four percent (29–40%) of patients experienced treatment-related acute toxicity necessitating a treatment interruption, the most common being skin related toxicity: 22% (17–27%) (). There were differences in the patterns of toxicity observed by age group: older patients were more likely to report diarrhea, with five patients aged 70 or over (Group 3) having significant diarrhea requiring treatment break compared to none in the other two groups.
Table III. Acute toxicity requiring treatment break.
Sensitivity analyses similarly confirmed a significant association of age with the development of diarrhea requiring a treatment break using a cut-off of > 75 years (Supplementary Table III available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513).
Tumor control and survival
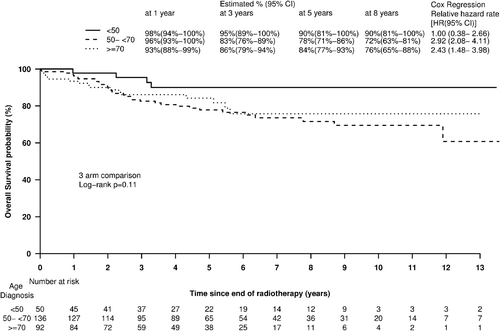
Tumor control and survival rates were analyzed for 278 patients with follow-up data. At a median follow-up of 5.3 (95% CI 5.0–6.1) years, there were 38 colostomies, 54 deaths, 43 locoregional and 17 distant recurrence events reported. Patterns of failure are outlined in and (Supplementary Table IV available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513). The five-year actuarial overall survival rates were 90% (81–100) for Group 1, 78% (71–86) for Group 2 and 84% (77–93) for Group 3 ().
Table IV. Patterns of recurrenceˆ and colostomy.
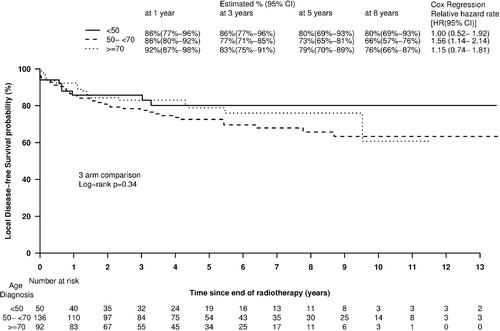
Age at diagnosis was not a significant predictor of overall survival when analyzed as a continuous (p = 0.15) or categorical (p = 0.25) variable on univariate analysis, nor when added to a multivariable model including T stage and sex. The five-year local recurrence free survival (LRFS) for Groups 1, 2, and 3 were 80% (69–93), 73% (65–81) and 79% (70–89), respectively (three-arm comparison log-rank p = 0.34) (). Corresponding percentages for five-year distant metastases-free survival were 92% (83–100), 83% (77–91) and 82% (74–91), respectively (p = 0.21). Age at diagnosis was not a significant predictor of either local- or distant-recurrence free survival, when analyzed as a continuous or categorical variable, in univariate or multiple variable models, nor when added to a multivariate model including T stage and sex (as categorical, p = 0.22).
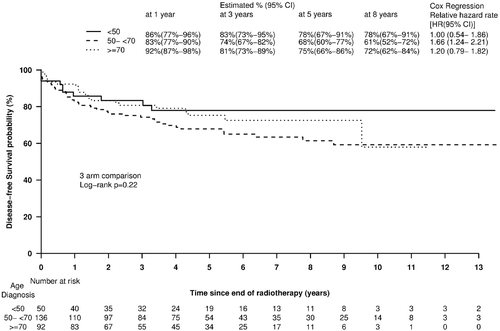
The five-year actuarial disease-free survival for Groups 1, 2 and 3 was 78 (67–91)%, 68 (60–77)% and 75 (66–86)%, respectively (p = 0.22) (). Age at diagnosis was not a significant predictor of disease-free survival when analyzed as a continuous (p = 0.70) or categorical (p = 0.94) variable on univariate and multivariate analysis (p = 1.00).
Post-hoc sensitivity analyses
Sensitivity analyses performed using varied age limits for Group 3, namely > 75 year olds and > 80 year olds are provided in (Supplementary Figures A1–A2, B1–B2, C1–C2 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013. 876513). Supplementary Figures B1–B2 and C1–C2 show there continues to be no significant effect of age on local recurrence free survival and disease-free survival, when cut-offs of > 75 or > 80 years are used.
(Supplementary Figure A1–A2 available online at http://informahealthcare.com/doi/abs/10.3109/ 0284186X.2013.876513) report p-values of 0.016 and 0.099 in the three-arm comparison when age cut-offs of > 75 and > 80 years are used, respectively. However, the 95% CI for each survival point- estimate considered as well as hazard ratios in these survival curves overlap each other. In addition the curves in (Supplementary Figure C2 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513) move in a different direction to those in and (Supplementary Figure C1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513).
Discussion
The significant toxicity of CRT for anal cancer presents challenges for oncologists when dealing with the elderly age group. When attempting to determine an older patient's fitness for aggressive treatment, clinicians are often faced with the uncertainty around basing treatment decisions on a patient's biological versus chronological age. Adding to this complexity is the inconsistent and varying definition of this so-called “elderly” age group. Whereas Japan [Citation6] and the US [Citation7] previously defined elderly as over 65 years, US Census data now define the > 65s as the “older population” [Citation8], a definition which is similarly used by the Australian Bureau of Statistics [Citation9].
It is worth noting that despite an increasing population in the > 65 and > 75 age group, they represent only about 14% and 5.8% of the total population, respectively. Thus, for the purposes of the current study, given the median-age at diagnosis for anal cancer is 60 years [Citation10], and to avoid the possibility of too few patient numbers in the later age group, an age-limit and cut-off of 70 years was selected at the planning of this study.
Our results show that elderly patients had comparable tumor control rates and outcome following curative CRT, with five- and eight-year disease-free survival rates of 75% [95% CI 66–86%] and 72% [95% CI 62–84%], respectively, which are similar to the five other studies reported so far [Citation11–15]. In order to determine if these results could vary depending on the specific age cut-off selected, we also performed post-hoc sensitivity analyses using upper age cut-offs of > 75 and > 80 years. As alluded to in the results section, these sensitivity analyses for local recurrence free survival and disease-free survival confirm the initial conclusions that there is no significant effect of age on these outcomes.
The curves for overall survival demonstrate a p-value of 0.016 when a cut-off of > 75 years was used (Supplementary Figure A1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513). These figures are interesting, as if the data were to suggest a difference in outcome with increasing age, one would expect the curves would move slightly apart, but in the same order and direction compared with the other age cut-offs. This is not demonstrated in the curves shown – the order of the curves changes for different age group cut-offs, and the uncertainty around each point estimate considered and hazard ratio suggests no evidence of a difference in outcome by age group. However, if the p-values alone are considered, as can be seen from (Supplementary Figure A1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.876513), the p-value of 0.016 suggests there may be a difference in overall survival at some time within 13 years from end of radiotherapy when using a cut-off of > 75 years. We are cautious about over-interpreting this significant p-value (in line with best practice in interpretation of results as suggested in Gardner and Altman [Citation16], as this interpretation is not supported by the other analyses using this cut-off. The confidence intervals for the point estimates of outcome rates at all time-points considered overlap each other, as do the confidence intervals for the hazard ratios. The suggestion of an association is also not supported with the sensitivity analyses with the alternate cut-offs for age group. Thus, our conclusion from this remains and supports that from the main analysis, that there is no evidence for a significant effect of age group on outcome.
Elderly patients also presented with similar stages of disease to that seen in the younger age groups, experienced similar rates of overall acute toxicity, and required no more frequent treatment breaks for toxicity. In this study, frequency of treatment break was selected as a surrogate endpoint for severe acute toxicity, where in practice these breaks would often be necessary for severe toxicity. For this cohort, no statistically significant difference in the proportion of patients requiring a treatment break was observed (39%, 28% and 33% of patients in the < 50, 50–70 and > 70 years age group, respectively). Likewise, the duration of unplanned break for each corresponding group was also similar at 10, 13 and 11 days, respectively (p = 0.36), the majority of which was due to skin toxicity. The acute toxicity reported in our cohort overall is comparable to that seen in RTOG 9811, which reported rates of grade 3 acute gastrointestinal (GI) and skin toxicity of 32–43% and 39–43%, respectively [Citation17].
Elderly patients seemed, however to have more severe acute gastro-intestinal toxicity, in particular, compared to younger patients. There were five patients in the > 70 years group whom developed diarrhea requiring a treatment break, compared to none in the other two groups. In addition, there was a greater proportion of patients in the > 70 years group (10%) whom failed to complete RT. The reasons for RT cessation were not reported in this study, however a possible explanation could be that the treating physicians may have potentially been more inclined to either institute a treatment break or even cease RT in the elderly age group. It is also worth noting that that the tumor control outcomes in the older age group are comparable despite a greater proportion of them not completing CRT. We postulate a possible explanation for this is that the total dose of 50.4–54 Gy used likely provides some “buffer”, in that the earliest studies by Nigro et al. [Citation18] demonstrated significant proportions of complete pathological responses even with doses as low as 30 Gy. Thus, it is possible (and/or likely) that a proportion of patients whom did not complete RT were potentially cured, despite receiving doses below 50.4 Gy. However, our data should not be interpreted so as to suggest that a lower radiation dose is always appropriate, as this would obviously require formal testing in a randomized fashion.
The retrospective nature of this analysis of course carries with it inherent biases and limitations, however, its strengths lie in the fact that, firstly, over the past 25 years, our institutional and unit policy has been to offer a uniform treatment regimen for anal cancer regardless of age, provided the patient is in good performance status and deemed to be fit for all components of the therapy. At the outset, at least, there were no planned modifications made in terms of 5-FU and MMC dosing and intensity. In addition, for the first 10 years or so, our center was the sole radiotherapy provider in the state of Victoria, as evidenced by the fact that the distribution of anal cancer across the age groups in this study is not dissimilar to that described in Australian population statistics.
In conclusion, our results show similar acute toxicity, disease control and overall survival at five years among different age groups. We would therefore advocate that advancing age alone should not preclude comprehensive assessment and staging, nor should it exclude curative standard CRT. It is likely that techniques such as IMRT would improve tolerability for all age groups without compromising tumor control.
Supplementary Tables I–IV
Download PDF (382.6 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Northover J, Glynne-Jones R, Sebag-Montefiore D, James R, Meadows H, Wan S, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 2010;102:1123–8.
- Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer. J Clin Oncol 1997;15:2040–9.
- Matzinger O, Roelofsen F, Mineur L, Koswig S, Van Der Steen-Banasik EM, Van Houtte P, et al. Mitomycin C with continuous fluorouracil or with cisplatin in combination with radiotherapy for locally advanced anal cancer (European Organisation for Research and Treatment of Cancer phase II study 22011-40014). Eur J Cancer 2009;45:2782–91.
- Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) books [Internet]. 2012 [cited 2012 Nov 29]. Available from: http://www.aihw.gov.au/acim-books/
- Tomaszewski JM, Link E, Leong T, Heriot A, Vazquez M, Chander S, et al. Twenty-five-year experience with radical chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys 2012;83:552–8.
- Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly”. Geriatr Gerontol Int 2006;6:149–58.
- Sixty-five plus in the United States [Internet]. United States Census Bur. 1995. Available from: http://www.census.gov/population/socdemo/statbriefs/agebrief.htm [cited 23 June 2013]
- Wan H, Sengupta M, Velkoff V, DeBarros K. US Census Bureau, Current Population Reports, 65 + in the United States: 2005. U.S. Government Printing Office: Washington, DC; 2005. p. 23–209.
- Australian Bureau of Statistics. 3201.0 Population by age and sex, Australian States and Territories [Internet]. 2010. Available from: http://www.abs.gov.au/AUSSTATS/[email protected]/Lookup/3201.0Main+Features1Jun2010?OpenDocument
- National Cancer Institute. SEER Cancer Statistics Factsheets: Anal Cancer [Internet]. Bethesda, MD. Available from: http://seer.cancer.gov/statfacts/html/anus.html
- Fallai C, Cerrotta A, Valvo F, Badii D, Olmi P. Anal carcinoma of the elderly treated with radiotherapy alone or with concomitant radio-chemotherapy. Crit Rev Oncol Hematol 2007;61:261–8.
- Valentini V, Morganti AG, Luzi S, Mantello G, Mantini G, Salvi G, et al. Is chemoradiation feasible in elderly patients? A study of 17 patients with anorectal carcinoma. Cancer 1997;80:1387–92.
- Charnley N, Choudhury A, Chesser P, Cooper RA, Sebag-Montefiore D. Effective treatment of anal cancer in the elderly with low-dose chemoradiotherapy. Br J Cancer 2005;92:1221–5.
- Allal A, Obradovic M, France L, Roth AD, Spada A, Marti M, et al. Treatment of anal carcinoma in the elderly. Feasibility and outcome of radical radiotherapy with or without concomitant chemotherapy. Cancer 1999;85:26–31.
- De Bari B, Chekrine T, Shakir IS, Ardiet JM, Gerard JP, Chapet O, et al. Should the treatment of anal carcinoma be adapted in the elderly? A retrospective analysis of toxicities in a French center. J Clin Gastroenterol 2011;45:738–9.
- Gardner MJ, Altman DG. Statistics in medicine confidence intervals rather than p values: Estimation rather than hypothesis testing. Br Med J 1986;292:746–50.
- Ajani J, Winter K, Gunderson L, Pedersen J, Benson AB, Thomas CR, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA 2008;299:1914–21.
- Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer 1983;51:1826–9.
- Salama JK, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity- modulated radiation therapy for anal canal cancer patients: A multicenter experience. J Clin Oncol 2007;25:4581–6.
- Milano MT, Jani AB, Farrey KJ, Rash C, Heimann R, Chmura SJ. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2005;63:354–61.
- Kachnic L, Winter K, Myerson R, Goodyear M, Willins J, Esthappan J, et al. RTOG 0529: A phase II evaluation of dose-painted IMRT in combination with 5-fluorouracil and mitomycin-C for reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013; 86:27–33.
- Brierley J, Dawson L, Sampson E, Bayley A, Scott S, Moseley JL, et al. Rectal motion in patients receiving preoperative radiotherapy for carcinoma of the rectum. Int J Radiat Oncol Biol Phys 2011;80:97–102.