Abstract
Background. The introduction of innovative non-invasive screening tests (e.g. tests based on stool and blood samples or both) may be a solution to increase colorectal cancer (CRC) screening uptake. However, preferences for these non-invasive screening tests have not been investigated in great detail yet. The purpose of this article therefore is to elicit individuals’ preferences for different non-invasive screening tests in a Dutch screening campaign context.
Material and methods. We investigate preferences by means of a labeled discrete choice experiment. Data of 815 individuals, aged 55–75 years, are used in the analysis.
Results. Multinomial logit model analysis showed that the combi-test is generally preferred over the blood-test and the (currently available) stool-test. Furthermore, besides the large effect of screening test type, there are significant differences in preference depending on participants’ socio-demographic background. Finally, the analysis showed a significant positive effect on screening test choice for the attributes sensitivity, risk reduction, and level of evidence and a non-significant effect for the attribute unnecessary follow-up test.
Conclusion. Introducing new non-invasive screening tests that are based on a combination of stool and blood samples (or blood sample only) has the potential to increase CRC screening participation compared to the current standard stool-based test.
It is of paramount importance to optimize colorectal cancer (CRC) screening participation as it helps to reduce the number of CRC deaths and morbidity [Citation1,Citation2]. Unfortunately, in many countries only a relatively small proportion of the targeted individuals participate in CRC screening. For example, several studies that used a Dutch population sample found that the participation rate in non-invasive CRC screening ranges from 49% to about 60% [Citation3,Citation4]. There are many barriers that restrain individuals from participating in CRC screening such as the discomfort and embarrassing nature of the test, limited knowledge about screening, the belief that screening is not effective, fatalism, the fear for finding cancer, and not wanting to know that something is wrong [Citation5–7]. In this paper we investigate individuals’ preferences for three non-invasive screening methods that address some of these barriers and therefore may help to increase screening participation. These non-invasive screening tests do not require bowel preparations and never lead to complications in contrast to more invasive methods such as sigmoidoscopy or colonoscopy. First, we consider the current standard screening test based on a stool sample. Second, we introduce a screening test that is new to the target population and based on a blood instead of a stool sample. This test may increase uptake of individuals that have a highly negative attitude towards the collection of stool samples [Citation6]. Third, we present a hypothetical “combination” test that is based on both a blood as well as a stool sample. This combi-test may be interesting as it has higher test accuracy (i.e. is more sensitive). Our idea for either the blood-test or combi-test is based on Bosch et al. [Citation8] who reviewed different molecular marker based tests in both blood and stool.
In sum, the introduction of new non-invasive screening tests that require different actions from individuals (e.g. taking a stool sample, blood sample, or a combination of both) and that have different accuracy levels may be a potential solution to the participation problem in campaign-based CRC screening. Yet, individuals’ preferences for such tests are unknown in a Dutch screening campaign context. Information about potential screening participants’ preferences and expected screening uptake is highly valuable as it may help policy makers in their decision whether or not to offer these tests in practice when they would become available for use. In this study, we elicit potential screening participants’ preferences for these new non-invasive screening tests and simulate choice shares and uptake when individuals would be offered these types of screening tests in a Dutch screening campaign context. More specifically, we provide an answer to the following research questions: 1) what are Dutch potential screening participants’ preferences regarding stool-, blood-, and combi-tests (i.e. is a more complex combi-test more attractive than a single test, and is a blood-test more attractive than a stool-test if accuracy is the same); 2) how do these preferences differ depending on participants’ socio-demographic characteristics; and 3) what role do test characteristics play in individuals’ participation decision?
Material and methods
Discrete choice experiment (DCE)
A discrete choice experiment (DCE) is a powerful technique to measure consumer preferences for goods and services [Citation9,Citation10] that is based on the random utility theory framework. The technique originates from marketing research, and was soon adapted for healthcare purposes such as the evaluation of healthcare interventions [Citation11]. It assumes that goods, services or interventions, like screening tests, can be defined by specific attributes (i.e. characteristics) and their levels (i.e. attribute levels). Furthermore, a DCE applies an experimental design that consists of a series of choice tasks in which two or more hypothetical goods, services or interventions are presented to respondents [Citation9,Citation10]. In each choice task respondents are asked to indicate their most preferred choice.
Selection of attributes and attribute levels
Attributes in the DCE are based on reviewing the literature on measuring preferences for CRC screening tests by means of discrete choice and conjoint analysis methods. The selected attributes (see ) are sensitivity (SENS), chance of unnecessary follow-up test (i.e. false-positive test result, UNNECESSARY FOLLOW-UP TEST), risk reduction of CRC death (RR), and scientific level of evidence (LEVEL OF EVIDENCE). The first three attributes are included because they are used most often in the reviewed studies [Citation12–22]. Furthermore, their importance was confirmed in structured interviews with Dutch experts in the field (n = 3). The fourth attribute – scientific level of evidence – indicates the strength of the scientific evidence available that is used to determine the levels for the attributes sensitivity, chance of unnecessary follow-up test and risk reduction. This is a relatively less commonly used attribute, but an understanding of the strength of evidence is highly important for healthcare decisions [Citation21,Citation23]. Marshall et al. [Citation24] argued that individuals’ may take into account the scientific evidence that supports CRC screening tests when they decide to participate in screening and that presenting this information may affect uptake. Furthermore, including this attribute is particularly relevant in this study as we also present respondents with screening tests for which available evidence is only limited.
Table I. Attributes, attribute descriptions, and attribute levels.
The levels of the attributes that are used are also based on a literature review (see above), the same experts’ opinions (n = 3), as well as on an analysis of CRC-related mortality-risk data. More specifically, we confirmed the correctness of the levels used by comparing them with an analysis of CRC-related mortality-risk data available at Erasmus MC, The Netherlands [Citation25] in which probabilities of CRC- related death are calculated given pre-specified levels of sensitivity and specificity. Attribute levels for the stool- and blood-tests differ from the attribute levels used for the combi-test to reflect that this latter test is based on a combination of the aforementioned tests (see next section) and thus has, for example, a higher likelihood of being more sensitive. For each attribute we used three levels except for scientific level of evidence which has two levels. For a more detailed description of the attributes and attribute levels used we refer to .
Experimental design
We created a labeled and blocked D-efficient design by using priors from an earlier DCE as part of the DeCoDe study [Citation26] and setting restrictions for specific attribute level combinations to promote realism (i.e. combinations for the attribute levels of sensitivity and risk reduction). The design was created in such a way that it allows for estimation of non-linear effects in the attribute levels. As two-way interaction effects were found to be non-significant in an earlier DCE [Citation26], these interactions were not incorporated in the current experimental design. Six blocks were used, each consisting of 12 choice tasks, to make the process of completing the survey not too burdensome for respondents [Citation27]. The restrictions are such that: 1) the lowest sensitivity level is presented only in combination with one of the two lowest levels of risk reduction; and 2) the highest sensitivity level is only presented in combination with the two highest risk reduction levels. Using these restrictions comes at the cost of level balance, but makes the experimental design more realistic. The labels used in the experimental design represent the base line utilities of the three different screening tests (a stool-test, blood-test, and combi-test). An option to indicate a preference not to participate in screening is also available to respondents. This “opt-out” option is included to reflect real-world screening practice where individuals may decide not to participate in screening. The stool-test involves taking a stool sample by means of a test kit at home and sending this test kit to the laboratory. The blood-test involves taking a blood sample at the hospital. Finally, the combi-test is a test based on a combination of the stool- and blood-tests. This involves taking both a stool sample at home, by means of a test kit, and a blood sample at the hospital. Using a labeled design, that presents the actual names of the screening tests, we are able to investigate potential screening participants’ preferences for these tests. Note that our explicit choice for the use of a labeled design reflects our particular interest in simulating choice shares and expected screening uptake. It is feasible because of individuals’ familiarity with the drawing of blood at the hospital and the collection of a stool sample at home also reflects a situation that – though novel in terms of the specific tests – closely reflects real life screening practice [Citation12].
Survey and sample
Based on the blocked design we created six different web-based surveys (e.g. see Supplementary Appendix A to be found online at http:// informahealthcare.com/doi/abs/10.3109/0284186X.2013.877159). In each survey respondents were told that the Dutch government had decided to start with a national screening campaign for the detection of CRC in 2013. This was followed by an explanation of the importance of CRC screening. Specifically, we clarified that the detection and removal of polyps helps to prevent CRC, and that the chance of survival increases when CRC is detected early. Furthermore, respondents were explained that there are different screening tests to detect polyps or CRC and that the survey was about their preferences for these tests. After this explanation they were informed about the different screening tests that can be used to detect CRC () and the attributes and attribute levels used to describe the different tests in the choice tasks. Respondents were also told that a (non- invasive) screening test does not give a definite result, but that the test only indicates if an additional follow-up test is necessary. This was followed by more detailed information about the follow-up test. Eventually, respondents were presented 13 choice tasks in which they were asked to imagine that they are invited to take part in the Dutch national screening campaign and that they have a choice between three different screening tests and an option not to participate in screening. Finally, respondents were asked to choose the test they preferred in each choice task. The first question was a warm-up question and is not used in the analysis. After completion of this question the actual choice tasks followed on the next 12 pages (one on each page, see for an example). All choice tasks were compulsory and respondents could only go on to the next page after having made a choice. When they finished the choice tasks respondents were asked some socio-demographic questions and thanked for their participation.
Table II. Non-invasive screening test information.
Table III. Choice task example*.
The surveys were placed on an online platform of a Dutch survey sampling solutions provider (SSI). Individuals were invited via e-mail to enter the platform and to complete our survey. Selection took place in such a way that the sample was representative for the Dutch screening population based on age (between 55 and 75 years) and gender (about equal percentage males/females). Eventually, 1575 individuals from the general Dutch population in the age category 55–75 years started our online survey in the second half of July 2012. Assignment to one of the six survey versions took place randomly. After completing the survey respondents were offered the option to donate a limited amount of money to charity for free as a compensation for their participation.
Econometric model
We analyzed the data using two multinomial logit (MNL) models, one without and one with socio-demographic characteristics in Nlogit 5.0. We included alternative specific parameters for the combi-test in the models since the attribute levels for the combi-test are different from the levels for the stool- and blood-tests. In the models, the utility function specification for a specific test, e.g. the stool-test (Ustool), consists of a systematic component (Vstool) and an error component (ϵstool). Below, the utility functions are described in more detail for the model without socio-demographic characteristics. In this model (β0, β8, β9) represent the alternative specific constants for the stool-, blood- and combi-tests, respectively. The attributes sensitivity, unnecessary follow-up test, and risk reduction of CRC death are coded as dummy variables that represent the level for a specific attribute with the highest level as a base (e.g. SENS50 is a dummy variable which is coded 1 if the test's sensitivity is 50 and 0 otherwise). Furthermore, level of evidence is a dummy variable coded 1 for strong evidence and 0 for limited evidence. The parameters of the aforementioned variables for the stool- and blood-tests are represented by β1–β7 and for the combi-test they are represented by β10–β16, respectively. The error components (ϵstool, ϵblood, ϵcombi) capture unexplained variation in preference and are assumed to be extreme value type I IID distributed. Note that the utility for choosing not to be screened (Unotest) is set to zero and used as a basis for comparison.
Results
Respondents
Of the 1575 individuals that started, 957 completed the survey (60.8%). From these completed survey versions we were able to use data of 815 respondents. More specifically, we deleted data from seven respondents because they turned out not to fall in the intended age category (i.e. 55–75 years), from 19 respondents because they currently were or had been CRC patients, and 122 respondents because they completed the survey in an amount of time that reflected that they had not taken the choice task seriously (less than 4 minutes). This completion time cut-off was discussed with and confirmed by the survey sample provider as a good indicator. In total 420 respondents were male (51.5%) and 395 female (48.5%). We present additional socio-demographic information of the data used in .
Table IV. Demographic characteristics.
DCE results
The model estimation results are provided in (models 1 and 2). The alternative specific constants (β0, β8, β9) indicate the general preference of individuals for a particular screening test relative to the option not to participate in screening. They are significant and have large positive values indicating that screening is preferred above no screening. The alternative specific constant for the combi-test (β9) has the highest value followed by the constants of the blood- and stool-tests, respectively. The differences between the tests are all significant in WALD tests of equal parameter values (p < 0.001) indicating a clear order in individuals’ screening test preference. In addition, we conducted a more detailed choice share simulation analysis to more clearly represent the size of this effect (see next section). Furthermore, we found significant negative effects for the sensitivity and risk reduction dummies (baseline is highest attribute level) and a positive effect of strong level of evidence (baseline is limited scientific evidence) for all screening tests. This indicates that as expected, more sensitive tests, tests that lead to a higher risk reduction of CRC death, and tests that are supported by strong scientific evidence are more likely to be chosen than less sensitive tests, tests that lead to a lower risk reduction of CRC death, and tests that are supported by limited scientific evidence. The parameters for the unnecessary follow-up test dummies are not significant for the blood-, stool- and combi-tests.
Table V. Model estimation results.
To demonstrate how screening test preferences are affected by individuals’ socio-demographic characteristics we estimated a second MNL model in which we included the characteristics education, gender, ethnicity, age, colonoscopy experience and screening test experience using alternative specific parameters for each test ( model 2). These characteristics are represented by the dummy coded variables high school, male, ethnicity, age, colonoscopy experience, and screening test experience. They are coded 1 if an individual's education level is higher or equal to high school, gender is male, ethnicity is Dutch (i.e. the individual is born in the Netherlands), an individual has experienced a colonoscopy in practice, and if an individual has screening test experience (and 0 otherwise). Furthermore, age is a continuous variable. We found that individuals that are higher educated (i.e. education level higher or equal to high school), born in the Netherlands, younger, and that have experienced a colonoscopy in practice are more likely to participate in either screening test than individuals that are lower educated (i.e. education level lower than high school), not born in the Netherlands, older, and that have not experienced a colonoscopy in practice. Furthermore, we found that women and individuals that have done a screening test before are more likely to participate in stool- and combi-tests than men, and individuals that have not done a screening test before.
Uptake simulation
We calculated test choice shares for several screening test scenarios by means of Nlogit's simulation procedure using the MNL model estimation results ( model 1). First, we predict initial choice shares (i.e. choice shares based on the sample data) of 16.8%, 26.3%, 43.3%, and 13.6% for the stool-, blood-, combi-tests, and opt-out option, respectively. These choice shares indicate that, on average, the combi-test is preferred, followed by the blood-test and then the stool-test.
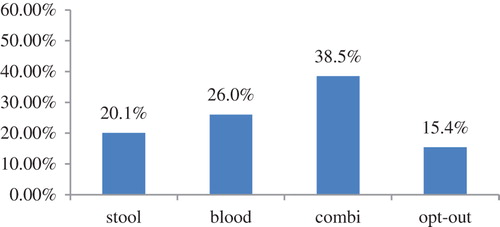
We also simulated choice shares when all three screening tests have the lowest utility (i.e. all three tests have the lowest sensitivity level, lead to the highest chance of an unnecessary follow-up test, have the lowest risk reduction level, and are supported by limited scientific evidence). In this case the choice shares are 13.5%, 22.5%, 38.1%, and 25.9% for the stool-, blood-, combi-tests and opt-out option, respectively. Furthermore, we simulated choice shares when all screening tests have the highest utility. In this case choice shares are 16.3%, 27.2%, 50.8%, and 5.73% for the stool-, blood-, combi-tests and opt-out option, respectively. Finally, in a screening test scenario where all three screening tests have an average utility (i.e. all three tests have an average sensitivity level, an average chance of an unnecessary follow-up test, an average risk reduction level, and are supported by limited scientific evidence) choice shares would be 16.4%, 27.3%, 40.3%, and 16.1% for the stool-, blood-, combi-tests and opt-out option, respectively. If the level of scientific evidence for the stool-test would be strong instead of limited in the latter simulation, choice shares would be 20.1%, 26.0%, 38.5%, and 15.4% (). These simulations confirm the target population's order of test preference (combi-test, blood-test, and stool-test) and indicate that in case three highest utility (i.e. best) screening tests are offered to individuals expected uptake would be 94.3%.
Discussion
Conclusion
In the introduction of the article we formulated three research questions: 1) what are Dutch potential screening participants’ preferences regarding stool-, blood-, and combi-tests (i.e. is a more complex combi-test more attractive than a single test, and is a blood-test more attractive than a stool-test if accuracy is the same); 2) how do these preferences differ depending on participants’ socio-demographic characteristics; and 3) what role do test characteristics play in individuals’ participation decision? Our answer to the first question is that the combi-test is generally preferred above the stool- and blood-tests and that, in case of a ‘single’ test, the blood-test is preferred over the stool-test. This result is particularly interesting for policy makers that aim to optimize uptake because a stool-based test (the least preferred test in our study) is currently used in Dutch screening camping practice.
Our answer to the second question is that the socio-demographic characteristics education, gender, ethnicity, age, colonoscopy experience, and screening test experience affect screening test choice. More specific, we found that individuals that are higher educated (high school or above), born in the Netherlands, younger, and that have experienced a colonoscopy in practice, are more likely to participate in either screening test, and that woman and individuals that have done a screening test before are more likely to participate in stool- and combi-tests.
Our answer to the third question is that the attributes sensitivity, risk reduction of CRC death, and scientific level of evidence have a significant positive effect on screening test choice and that the attribute ‘chance of an unnecessary follow-up test’ has no significant effect. Sensitivity and risk reduction of CRC death are the most important attributes. However, despite their importance, screening test type (i.e. the label) is most influential in screening test choice.
Limitations and further research
In this paragraph we present several limitations and possible considerations that may be interesting for future research.
A first limitation deals with the description of the different tests used in the DCE. We described the stool-test as: “At home you collect a small sample of your stool by using a test kit. Then, you send the test kit back to the lab by post. If abnormalities are found, you are advised to do a follow-up test”. Whereas the blood-test is described as: “In the hospital a tube of blood is drawn by means of a blood-test. This will be sent to the lab for analysis. If abnormalities are found, you are advised to do a follow-up test”. In the stool-test description there is no information about possible costs of sending the test kit to the lab, furthermore in the blood-test description there is no information given that informs individuals about the waiting time at the hospital. Presenting this additional information can be important as its inclusion may affect individuals’ preferences for these screening tests. A related and interesting idea for future research would therefore be to test individuals’ preferences for stool-, blood-, and combi-tests using more detailed test descriptions. It may also be interesting to investigate if preferences would change if the drawing of blood for a blood-test can be done at different locations (e.g. also at a general practitioner's office instead of a hospital only).
A second limitation of our study is that the study set-up does not perfectly reflect current screening practice. Uptake percentages found may therefore differ from actual uptake percentages. Specifically, in the Dutch CRC screening campaign that starts in 2013 individuals will be invited to participate in a stool-test (i.e. iFOBT) and are not offered a choice between screening tests, whereas in our study individuals were presented a choice between three non-invasive screening tests (and the option not to participate in screening). Therefore, our study set-up does not allow for more realistic uptake analyses in which individuals are only offered one standard screening test (e.g. the current standard stool-test). If we would have chosen a set-up in which individuals were not offered a choice between current and hypothetical innovative screening tests, but were only asked whether they would participate given the specific screening test offered, more realistic uptake analyses would have been possible. However, in this case it would have been very difficult to derive a relative order of preference for the three non-invasive screening tests and to determine the relative importance of the attributes of the screening tests. This information can best be derived from a DCE in which individuals are presented a choice between multiple screening test options – as has been done in our study – because they would be forced to make direct comparisons between different screening tests. Nevertheless, it would be an interesting idea for future research to also perform an experiment with a set-up that offers no choice between screening tests and to compare the results with our study.
A third limitation, also related to the procedure of the test, is that we did not specify the screening interval (i.e. we have not told respondents that FOBT screening is usually repeated every two years). Furthermore, we did not provide information about how the test results are communicated to patients. However, in earlier research the variable transmission of test result (i.e. by mail, gastroenterologist, or physician) was found to be non-significant [Citation19]. Therefore it was omitted from the current task.
Fourth, the fact that the choice tasks presented to respondents were relatively complex can be considered as another limitation. We used a large amount of (probability) information to present the attributes and attribute levels in a choice task. The reason for this was to present the information of the attributes and attribute levels as precise as possible.
Fifth, about 56% of the respondents reported to know someone who has (had) CRC. This seems to be a large percentage and may be an indication of sample bias for CRC-related experiences. However, this high percentage can also be explained by the fact that CRC is one of the most common cancers in individuals aged above 55. Furthermore, it may be the case that individuals considered many people in their environment as ‘friends/acquaintances’ leading to a high percentage of individuals in this category. The finding that almost 30% of the individuals in our sample had undergone a colonoscopy may be explained by the possibility that individuals did not distinguish between flexible sigmoidoscopy and colonoscopy due to barriers in their knowledge about CRC screening [Citation5].
Finally, we conclude by noting that DCEs reveal stated preferences and that individuals’ actual participation decision (or screening test choice) may be different if the stool-, blood-, and combi-tests would be offered in a real life setting. Due to the fact that our DCE involves hypothetical choices (i.e. the blood- and combi-tests do not represent current screening practice) and the fact that stated preferences (SP) and revealed preferences (RP) data may differ [Citation28], decision makers should be cautious when they use the choice shares and choice share changes from our analysis to support decision making. Specifically, our results have limited value for predicting the uptake of the current standard stool-test in the actual Dutch screening campaign due to the presence of the hypothetical screening tests in the choice tasks. However, the derived choice shares indicate the target populations’ relative order of preference for the hypothetical screening tests in comparison with the current standard stool-test, and thus provide information about the potential of these screening tests in future CRC screening campaigns based on population preferences. A final possibility for future research would therefore be an analysis of direct comparisons to actual choices made in the Dutch screening campaign and the results from our analysis. It would be highly interesting to compare and maybe combine RP and SP data [Citation28] and investigate possible discrepancies between the two.
Theoretical and managerial implications
With this paper we build on the existing literature that focuses on measuring individuals’ test preferences to screen for CRC by means of discrete choice experimentation. As far as we know, this is the first DCE-based study that investigates Dutch individuals’ screening test preferences for non-invasive screening tests that are based on samples of blood, stool, or a combination of these. Despite that a stool-based test (iFOBT) will be used in the Dutch CRC screening campaign that starts in 2013, our findings illustrate a clear preference of the general public for other non-invasive screening tests such as blood- and combi-tests. Although theoretically interesting, this finding also has practical value as it can be used to support research on new molecular marker based screening tests for use in future CRC screening campaigns. According to our findings more emphasis should be placed on an investigation of the clinical value of new molecular markers for the detection of CRC in blood as well as stool samples, as the use of accurate blood- and combi-tests would most likely increase screening participation. However, one should note that this finding cannot be generalized as it may be different in other countries. Nayaradou et al. [Citation19], for example, found that the French general public prefers tests based on a stool sample instead of a blood sample. One explanation for this may be the existing cultural differences between countries. Another explanation may be the difference between samples used. In our study individuals have, on average, less experience with CRC screening. We found that individuals are more likely to prefer the stool-test above the blood-test when they have screening test experience, possibly due to endowment effects or status quo bias [Citation29]. This difference in individuals’ CRC screening experience may thus explain why stool-tests are preferred above blood-tests in Nayaradou et al. [Citation19], but not in our study. Furthermore, the question remains whether blood-tests, that are as accurate as or more accurate than the current standard stool-test, can actually be developed in practice. With this in mind, another practical suggestion would be to positively affect consumer preferences for the stool-test using, for example, marketing strategies that clarify the benefits of stool-based testing to the general public. This is particularly relevant given that stool-tests will be used in the current Dutch screening campaign and that this type of test is also likely to be used in future screening practice.
The finding that, in their screening participation decision, individuals put most attention to the screening test (label) (i.e. blood-, stool-, or combi-tests) rather than the characteristics of the screening test can be used by policy makers in practice. They could, for instance, emphasize the type of screening test(s) used in screening invitation flyers and focus less on the explanation of the test's characteristics. This finding is similar to de Bekker-Grob et al. [Citation12] in which results of a labeled and unlabeled DCE are compared. The authors found that reduced attention was given to attributes in a labeled (versus unlabeled) design and illustrated that a large percentage of respondents (approximately 22%) had dominant preferences for the screening test labels. This was also the case in our study in which approximately 33% of individuals had dominant preferences for a specific type of screening test.
Based on market share calculations on the sample data approximately 86% of the individuals would take part in screening. This participation rate is somewhat higher than in other Dutch DCE-based studies. For example, van Dam et al. [Citation22] found that uptake would be 72% for FOBT screening given a 10% risk reduction of CRC-related death. Furthermore, Hol et al. [Citation15] found uptake rates of 68% for FOBT and 77% for colonoscopy screening on the basis of realistic screening intervals and a mortality reduction level adapted from the literature. One explanation for the somewhat higher participation rates in this study may be the specific type of DCE used, that is, our DCE is labeled instead of unlabeled [Citation12] and does not offer individuals a direct choice for an invasive follow-up test (colonoscopy). Another explanation may be based on the fact that, in our DCE, individuals are offered a choice between three different screening tests, and therefore have a higher chance to get a screening test for which they would be willing to participate. In practice decision makers may use this concept of customized care (i.e. not only offering a standard screening test which is the same for everyone, but offering individuals a choice between blood-, stool-, and combi-tests) as this may increase screening participation.
Supplementary Appendix A
Download PDF (41 KB)Acknowledgements
Comments by seminar participants in presentations at the institute of Health Policy & Management (iBMG) and the annual DeCoDe and CTMM meetings (2011 and 2012) are highly appreciated. Furthermore, we thank Marjolein van Ballegooien, Esther de Bekker-Grob, and Luuk Goede for their interview and assistance with the selection of attributes and attribute levels and Manon van Engeland and Veerle Melotte for clarifying screening test information. We also thank Gerrit Meijer and Carin Uyl-de Groot for their helpful suggestions.
Declaration of interest: The views expressed and any flaws remain the full responsibility of the authors. This study was performed within the framework of CTMM, the Center for Translational Molecular Medicine. DeCoDe project (grant 03O-101). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mandel JS, Bond JH, Church TR, Dale C, Snover G, Bradley M, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993; 328:1365–71.
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale – Update based on new evidence. Gastroenterology 2003;124:544–60.
- Deutekom M, van Rijn AF, Dekker E, Blaauwgeers H, Stronks K, Fockens P, et al. Uptake of faecal occult blood-test colorectal cancer screening by different ethnic groups in the Netherlands. Eur J Public Health 2009;19:400–2.
- van Rossum LG, van Rijn AF, Laheij RJ, Van Oijen MG, Fockens P, van Krieken HH, et al. Random comparison of guaiac and immunochemical fecal occult blood-tests for colorectal cancer in a screening population. Gastroenterology 2008;135:82–90.
- Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc 2008;56:307–14.
- Jones RM, Woolf SH, Cunningham TD, Johnson RE, Krist AH, Rothemich SF, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med 2010;38:499–507.
- Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening. A comparison of reports from primary care physicians and average-risk adults. Med Care 2005;43:939–44.
- Bosch LJW, Carvalho B, Fijneman RJA, Jimenez CR, Pinedo HM, van Engeland M, et al. Molecular tests for colorectal cancer screening. Clin Colorectal Cancer 2011;10:8–23.
- Hensher DA, Rose JM, Greene WH. Applied choice analysis: A primer. Cambridge: Cambridge University Press; 2005.
- Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care (vol. 11). Dordrecht, the Netherlands: Springer; 2008.
- De Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: A review of the literature. Health Econ 2012;21:145–72.
- De Bekker-Grob EW, Hol L, Donkers B, van Dam L, Habbema JDF, van Leerdam ME, et al. Labeled versus unlabeled discrete choice experiments in health economics: An application to colorectal cancer screening. Value Health 2010;13:315–23.
- Gyrd-Hansen G, Søgaard J. Analysing public preferences for cancer screening programmes. Health Econ 2001; 10:617–34.
- Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care 2008;46:10–16.
- Hol L, de Bekker-Grob EW, van Dam L, Donkers B, Kuipers EJ, Habbema JDF, et al. Preferences for colorectal cancer screening strategies: A discrete choice experiment. Br J Cancer 2010;102:972–80.
- Howard K, Salkeld G. Does attribute framing in discrete choice experiments influence willingness to pay?. Results from a discrete choice experiment in screening for colorectal cancer. Value Health 2009;12:354–63.
- Marshall DA, Reed Johnson F, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health 2007;10:415–30.
- Marshall DA, Reed Johnson, F, Kulin NA, Özedmir S, Walsh JME, Marshall JK, et al. How do physician assessments of patient preferences for colorectal cancer screening tests differ from actual preferences? A comparison in Canada and the United States using a stated-choice survey. Health Econ 2009;18:1420–39.
- Nayaradou M, Berchi C, Dejardin O, Launoy G. Eliciting population preferences for mass colorectal cancer screening organization. Med Decis Making 2010;30:224–33.
- Ryan M, San Miguel F. Revisiting the axiom of completeness in health care. Health Econ 2003;12:295–307.
- Salkeld G, Solomon, M, Short L, Ryan M, Ward JE. Evidence-based consumer choice: A case study in colorectal cancer screening. Austr N Z J Public Health 2003;27: 449–55.
- Van Dam L, Hol L, de Bekker-Grob EW, Steyerberg EW, Kuipers EJ, Habbema JDF, et al. What determines individuals’ preferences for colorectal cancer screening programmes?. A discrete choice experiment. Eur J Cancer 2010;46:150–9.
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches. The GRADE working Group. BMC Health Serv Res 2004;4:38.
- Marshall DA, McGregor ES, Currie G. Measuring preferences for colorectal cancer screening. What are the implications for moving forward? Patient 2010;3: 79–89.
- Goede SL, van Roon AH, Reijerink JC, van Vuuren AJ, Lansdorp-Vogelaar I, Habbema JD, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut 2013;62:727–34.
- Benning TM, Dirksen CD, Dellaert BGC, Severens JL. Measuring consumers’ preferences for colorectal cancer screening tests in The Netherlands. Unpublished observations.
- Bech M, Kjaer T, Lauridsen J. Does the number of choice sets matter?. Results from a web survey applying a discrete choice experiment. Health Econ 2011;20:273–86.
- Ben-Akiva M, Bradley M, Morikawa T, Benjamin J, Novak T, Oppewal H, et al. Combining revealed and stated preferences data. Market Lett 1994;5:335–49.
- Salkeld G, Ryan M, Short L. The veil of experience: Do consumers prefer what they know best?. Health Econ 2000;9:267–70.