To the Editor,
According to the Dutch clinical practice guideline, the standard adjuvant treatment for patients with stage III colon cancer is a combination of fluorouracil, leucovorin and oxaliplatin (FOLFOX). Fluorouracil can be replaced by its oral analogue capecitabine, which provides similar overall survival, and is more convenient for the patient. The guideline also states that an adjuvant regimen without oxaliplatin, i.e. capecitabine monotherapy, can be considered in cases of advanced age or concomitant comorbidity. The efficacy of these chemotherapy schemes has been established in clinical trials, which showed improved disease-free and overall survival [Citation1–3].
Population-based studies have shown that the administration of chemotherapy declines with increasing age [Citation4–6]. For individual patients, there may be valid reasons to deviate from standard treatment, however, variations in treatment between hospitals or geographic regions after adjustment for patient casemix indicate that arguments for deviating from clinical guidelines are interpreted differently across hospitals [Citation5–8].
To date, the type of chemotherapy has often been ignored in population-based studies. Therefore, the current study evaluates which factors influence the administration of oxaliplatin-based chemotherapy as compared to non-oxaliplatin-based chemotherapy for patients with stage III colon cancer treated in daily clinical practice.
Patients and methods
Data from the Eindhoven Cancer Registry (ECR) were used. The ECR is a population-based registry that collects data on all newly diagnosed cancer patients in the southern Netherlands. The registry area comprises about 2.4 million inhabitants and encompasses six pathology departments, 10 community hospitals, and two radiotherapy institutions. Information on patient and tumour characteristics, diagnosis and treatment is routinely extracted from the medical records by trained administrators. The anatomical site of the tumour is registered according to the International Classification of Disease – Oncology (ICD-O). The tumour-node-metastasis (TNM) classification is used for stage notification of the primary tumour, according to the edition valid at the time of cancer diagnosis. Socioeconomic status (SES), based on individual fiscal data on the economic value of the home and household income, is provided at an aggregated level for each postal code. Comorbidities are registered according to a slightly modified version of the Charlson Comorbidity Index.
For the present study, patients who underwent resection only or who underwent resection and received adjuvant chemotherapy for primary colon cancer stage III (TanyN1–2M0) diagnosed between 2008 and 2011 were included. Patients were excluded if they had received radiotherapy (n = 20), neo- adjuvant chemotherapy (n = 6), local chemotherapy (n = 3) or targeted therapy (n = 7). Stage was based on the pathological TNM classification. Tumour localisation was categorised into three subsites: proximal colon (C18.0–C18.5), distal colon (C18.6– C18.7) and colon other/not otherwise specified (C18.8–C18.9). Patients were divided into age groups: < 70, 70–74 and ≥ 75 years.
Statistical analyses
Differences in patient and tumour characteristics between patients receiving oxaliplatin-based chemotherapy, non-oxaliplatin-based chemotherapy and no chemotherapy were analysed using χ2-tests. Differences in casemix between the hospitals were also analysed using χ2-tests. Patients with unknown SES, comorbidity, T stage, subsite or differentiation grade were not excluded from the study population, but were not taken into account in the respective χ2 calculations.
After stratification by age group, the observed proportions of patients receiving oxaliplatin and non-oxaliplatin-based chemotherapy were calculated for each hospital. The expected proportions of patients receiving oxaliplatin and non-oxaliplatin-based chemotherapy were also calculated after stratification by age group: a multivariable logistic regression model was used to calculate the chance of an individual patient receiving oxaliplatin and non-oxaliplatin-based chemotherapy, adjusted for gender, age, SES, comorbidity, T stage, N stage, subsite, differentiation grade and period of diagnosis. Based on these individual chances, the chance of receiving oxaliplatin and non-oxaliplatin-based chemotherapy was calculated for each hospital by taking the mean of all chances of the individual patients of each hospital.
After exclusion of patients who did not receive adjuvant chemotherapy, multivariable logistic regression analysis was conducted to assess the influence of several patient and tumour characteristics and hospital on the probability of the administration of oxaliplatin.
P-values below 0.05 were considered statistically significant. SAS/STAT® statistical software (SAS system 9.3, SAS Institute, Cary, NC, USA) was used for all analyses.
Results
The study population consisted of 1140 patients. Overall, 47% of the patients received oxaliplatin-based chemotherapy, 13% received non-oxaliplatin-based chemotherapy and 40% received no chemotherapy. shows the general characteristics of the study population by adjuvant treatment.
Table I. Characteristics of patients with resected stage III colon cancer, by adjuvant treatment (n = 1140).
With regard to casemix in the hospitals, SES and N stage differed significantly between the hospitals (p = 0.007 and p = 0.048, respectively). Gender, age, comorbidity, subsite, differentiation grade and period of diagnosis did not differ significantly between hospitals.
shows the observed and expected proportions of patients receiving oxaliplatin and non-oxaliplatin-based chemotherapy by hospital of treatment and stratified according to age group.
Figure 1. Observed and expected proportions of patients receiving oxaliplatin-based chemotherapy (dotted dark grey) and non- oxaliplatin-based chemotherapy (shaded light grey) among patients with resected stage III colon cancer, by hospital of treatment and age group; expected proportions based on casemix adjustment for gender, age, SES, comorbidity, T stage, N stage, subsite, differentiation grade and period of diagnosis (A: n = 531; B: n = 175; C: n = 434).
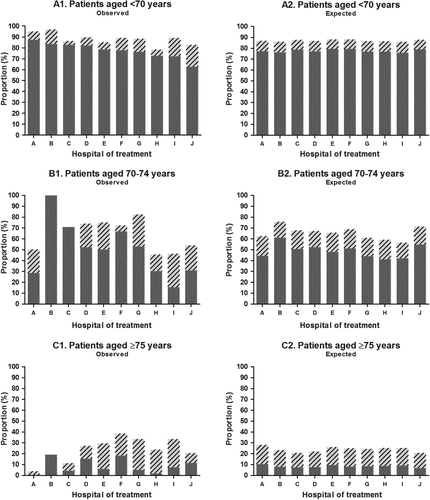
For patients aged < 70 years, the observed proportions of patients receiving oxaliplatin varied from 63% to 87% between the different hospitals (Δ = 24%), whereas the expected proportions of patients receiving oxaliplatin ranged from 76% to 79% (Δ = 3%). Similarly, the observed proportions of patients receiving non-oxaliplatin varied between 3% and 20% (Δ = 17%), whereas the expected proportions ranged from 9% to 10% (Δ = 1%).
In patients aged 70–74 years, the observed proportions receiving oxaliplatin varied from 15% to 100% between hospitals (Δ = 85%), whereas the expected proportions ranged between 41% and 61% (Δ = 20%). Observed proportions receiving non-oxaliplatin ranged from 0% to 29% (Δ = 29%), whereas expected proportions varied between 14% and 18% (Δ = 4%).
For patients aged ≥ 75 years, observed proportions of patients receiving oxaliplatin ranged from 0% to 19% (Δ = 19%), whereas the expected proportions varied between 7% and 10% (Δ = 3%). With respect to patients receiving non-oxaliplatin, observed proportions ranged between 0% and 28% (Δ = 28%), whereas the expected proportions varied from 13% to 18% (Δ = 5%).
In the subgroup of patients treated in the adjuvant setting, large inter-hospital variation in the addition of oxaliplatin was noted, ranging from 76% to 96% for patients aged < 70 years, from 33% to 100% for patients aged 70–74 years, and from 0% to 100% for patients aged ≥ 75 years.
presents the crude percentages and adjusted odds ratios for receipt of oxaliplatin among patients treated with adjuvant chemotherapy. Patients treated in hospital G and I were less likely to receive oxaliplatin than patients treated in hospital C (adjusted OR for G vs. C 0.35, 95% CI 0.14–0.85; adjusted OR for I vs. C 0.26, 95% CI 0.10–0.69). Furthermore, older patients, patients with a T1 stage and patients diagnosed in 2008– 2009 were also less likely to receive oxaliplatin.
Table II. Crude percentages and odds ratiosa for receipt of oxaliplatin-based adjuvant chemotherapy among patients with stage III colon cancer treated with adjuvant chemotherapy (n = 678).
Discussion
The current study showed that although the casemix was similar in all hospitals and the expected proportions of patients receiving oxaliplatin therefore varied little between hospitals, the observed proportions of patients receiving oxaliplatin varied greatly between hospitals. In the multivariate analysis, patients from two hospitals had decreased odds of receiving oxaliplatin. Although the addition of oxaliplatin improves disease-free and overall survival, this improvement is rather limited [Citation2,Citation9]. Additionally, the administration of oxaliplatin is associated with more toxicity and more inconvenience due to the need for intravenous administration [Citation3]. These factors are likely taken into account by medical oncologists. A study recently found that physician and hospital characteristics had little influence on the receipt of adjuvant oxaliplatin among patients aged ≥ 65 years [Citation10]. However, another study found that patients in rural regions were less likely to receive oxaliplatin-containing chemotherapy than patients from large metropolitan regions [Citation11]. The wide variation among hospitals in chemotherapy administration and type of chemotherapy demonstrated in the present study underscores the influence of institutional factors and local practice patterns in determining the administration and type of adjuvant chemotherapy. Data on the influence of physicians and practice settings on the prescription of adjuvant systemic treatment in general are scarce. In 2009, a population-based study showed that elderly women with breast cancer treated by oncologists in a private practice were more likely to receive adjuvant chemotherapy. This was presumed to be related to patient volume; oncologists in private practices generally had a higher patient volume, translating into a greater comfort level in treating patients [Citation12]. Other factors hypothesised by the authors to play a role in daily practice are clinical tradition, age of the medical specialists, multidisciplinary team meetings, involvement of medical specialists in scientific research and the teaching status of the hospital.
The range of observed proportions of patients receiving oxaliplatin among hospitals was found to vary more for patients aged ≥ 75 years than for patients aged < 70 years. Despite the significant proportion of elderly patients with colon cancer in clinical practice, the elderly are often excluded from clinical trials. Approximately 50% of patients with colon cancer are aged ≥ 70 years, but only 16% of patients enrolled in trials were ≥ 70 years [Citation13]. Since data on the effects of adjuvant chemotherapy in patients aged ≥ 75 years is scarce, it is impossible for guidelines to advise on adjuvant chemotherapy for patients over 75 years of age and even more complicated to indicate who is eligible for platinum-based regimens, leaving this decision up to the physicians’ judgement. However, a pooled analysis using data from randomised trials compared the efficacy of adjuvant chemotherapy for elderly and non-elderly patients and found that the efficacy of FOLFOX with regard to disease-free and overall survival was similar for patients aged less than 70 years and patients aged 70 years or older [Citation13]. This confirms that older patients fit enough to meet clinical trial eligibility criteria derive the same benefit from adjuvant chemotherapy as younger trial participants. However, whether the addition of oxaliplatin offers additional benefit to patients aged ≥ 75 years remains unclear, as the available data are conflicting [Citation9,Citation13–16]. A previous study suggested that physicians consider the eligibility criteria of the MOSAIC trial (i.e. age < 75 years, Karnofsky performance score ≥ 60, and adequate blood counts and liver and kidney function) when deciding on the prescription of oxaliplatin [Citation17]. Uncertainty remains over whether the lower rates of oxaliplatin observed in elderly patients represent justified clinical judgement or undertreatment. Moreover, this observational study cannot answer to what degree the older patients not treated adjuvantly might also have tolerated and benefited from the administration of adjuvant chemotherapy.
A limitation of our study is that detailed information on patients’ health status and nutritional status is lacking. ECOG performance status, for example, has previously been demonstrated to influence the administration of oxaliplatin [Citation18]. However, the results of our study indicate that casemix did not differ between hospitals so this cannot explain the large variation in adjuvant (oxaliplatin-based) chemotherapy administration found between hospitals. Another limitation of our study is that for those patients receiving adjuvant chemotherapy, additional information on the number of (oxaliplatin-based) cycles administered, dose schedules used and dose modifications were not available. Previous research showed that approximately 30% of stage III colon cancer patients discontinued adjuvant therapy after less than three months despite recommendations to continue for six months [Citation18]. Additionally, older patients are more likely than their younger counterparts to discontinue oxaliplatin early [Citation19].
In conclusion, the decision to administer oxaliplatin does not only depend on predictable factors such as age and T stage, but also on the hospital. The impact of hospital variation in type of adjuvant chemotherapy on outcomes such as toxicity, disease-free survival and quality of life needs to be further elucidated in population-based studies.
Acknowledgements
The authors thank the registration team of the Eindhoven Cancer Registry for their dedicated data collection. Furthermore, we would like to thank all the contributing hospitals: Amphia Hospital, Breda; Catharina Hospital, Eindhoven; Elkerliek Hospital, Helmond and Deurne; Hospital Bernhoven, Uden; Jeroen Bosch Hospital, ‘s Hertogenbosch; Máxima Medical Centre, Eindhoven and Veldhoven; St. Anna Hospital, Geldrop; St. Elisabeth Hospital, Tilburg; TweeSteden Hospital, Tilburg and Waalwijk; VieCuri Hospital, Venlo and Venray; Instituut Verbeeten, Tilburg.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by The Netherlands Organisation for Health Research and Development [grant number 113102004]. The funding source had no involvement in the study design, the collection, analysis and interpretation of data; in writing of the manuscript; and in the decision to submit the manuscript for publication.
References
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. New Engl J Med 2005;352: 2696–704.
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
- Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol 2007;25:2198–204.
- Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 2001;93:850–7.
- van Steenbergen LN, Rutten HJ, Creemers GJ, Pruijt JF, Coebergh JW, Lemmens VE. Large age and hospital-dependent variation in administration of adjuvant chemotherapy for stage III colon cancer in southern Netherlands. Ann Oncol 2010;21:1273–8.
- van Steenbergen LN, Lemmens VE, Rutten HJ, Wymenga AN, Nortier JW, Janssen-Heijnen ML. Increased adjuvant treatment and improved survival in elderly stage III colon cancer patients in The Netherlands. Ann Oncol 2012;23:2805–11.
- Chagpar R, Xing Y, Chiang YJ, Feig BW, Chang GJ, You YN, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol 2012;30:972–9.
- Panchal JM, Lairson DR, Chan W, Du XL. Geographic variation and sociodemographic disparity in the use of oxaliplatin-containing chemotherapy in patients with stage III colon cancer. Clin Colorectal Cancer 2013;12:113–21.
- Sanoff HK, Carpenter WR, Martin CF, Sargent DJ, Meyerhardt JA, Sturmer T, et al. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J Natl Cancer Inst 2012;104:211–27.
- Lund JL, Sturmer T, Sanoff HK, Brookhart A, Sandler RS, Warren JL. Determinants of adjuvant oxaliplatin receipt among older stage II and III colorectal cancer patients. Cancer 2013;119:2038–47.
- Panchal JM, Lairson DR, Chan W, Du XL. Geographic variation and sociodemographic disparity in the use of oxaliplatin-containing chemotherapy in patients with stage III colon cancer. Clin Colorectal Cancer 2013;12:113–21.
- Hershman DL, Buono D, McBride RB, Tsai WY, Neugut AI. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer 2009;115:3848–57.
- Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
- Sanoff HK, Carpenter WR, Sturmer T, Goldberg RM, Martin CF, Fine JP, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34.
- Hsiao FY, Mullins CD, Onukwugha E, Pandya N, Hanna N. Comparative effectiveness of different chemotherapeutic regimens on survival of people aged 66 and older with stage III colon cancer: A “real world” analysis using Surveillance, Epidemiology, and End Results-Medicare data. J Am Geriatr Soc 2011;59:1717–23.
- Tournigand C, Andre T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: Subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
- van Gils CW, Koopman M, Mol L, Redekop WK, Uyl-de Groot CA, Punt CJ. Adjuvant chemotherapy in stage III colon cancer: Guideline implementation, patterns of use and outcomes in daily practice in The Netherlands. Acta Oncol 2012;51:57–64.
- Abrams TA, Brightly R, Mao J, Kirkner G, Meyerhardt JA, Schrag D, et al. Patterns of adjuvant chemotherapy use in a population-based cohort of patients with resected stage II or III colon cancer. J Clin Oncol 2011;29:3255–62.
- Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
