To the Editor,
Chordomas are rare tumors that arise from the vestigial or ectopic notochord, with an incidence of 0.5 to 0.8 per 1 000 000 [Citation1,Citation2]. About 50% originate from the sacrococcygeal region, 30% from the skull base and 15% from vertebral bodies [Citation3]. A complete surgical resection is still considered the gold standard for treatment, when feasible. When this is not possible, high dose radiotherapy is often recommended [Citation4]. Medical treatment has also been evaluated, but has mainly been reported as case studies. The rationale for using drugs that interact with epidermal growth factor receptor (EGFR) is accumulating preclinical evidence that EGFR signaling is important in chordoma [Citation5–7]. A few case reports have also indicated that EGFR inhibition may be of value in treating chordomas [Citation8–11]. Although reliable data is limited, some reports support the hypothesis that regulators of angiogenesis, such as vascular endothelial growth factor (VEGF) and HIF-1α [Citation8,Citation12,Citation13], play a role in the development of chordoma. In addition, imatinib has demonstrated clinically interesting effects in locally advanced tumors that express platelet-derived growth factor β (PDGFB) and/or its receptor (PDGFRB) [Citation14].
We report here the effects of erlotinib and bevacizumab in three consecutive patients with chordoma. While the clinical and neuroradiological characteristics were consistent with the diagnosis in one of the cases (Case 1), the diagnosis had been confirmed by histopathology in the remaining two cases (Cases 2 and 3).
Case 1
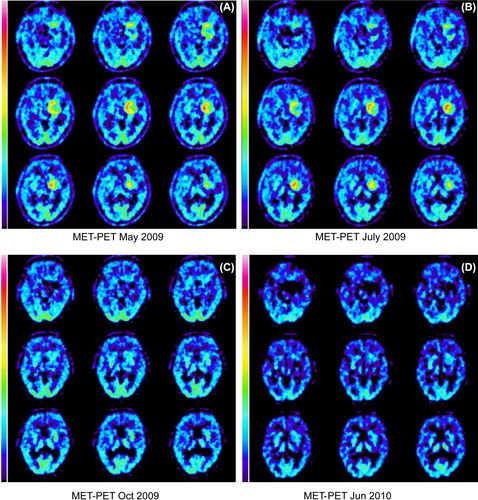
We have described this case previously [Citation8] and here supplement the data with a longer follow-up time. Briefly, a male patient aged 63 when he was diagnosed in 2007 with a skull-base tumor that involved the clivus and dorsum sellae. An attempt to resect the tumor was complicated by severe life-threatening bradycardia as soon as the neurosurgeon manipulated the dura. Thus, no tissue-based diagnosis was available before radiotherapy, which was given between December 2007 and January 2008, and included fractionated stereotactic radiotherapy followed by a proton boost. Magnetic resonance imaging (MRI) follow-up showed disease progression, while an 11C-methionine positron emission tomography (PET) scan in May 2009 showed enhanced uptake in the tumor (). Treatment with erlotinib and cetuximab was started the same month and had no significant effect on methionine uptake. Indeed, a slight increase in uptake occurred in a PET in July 2009 (). Cetuximab was replaced by bevacizumab in July, while erlotinib treatment continued. Interestingly, methionine uptake decreased significantly to slightly below normal in October 2009 () and this level had persisted in a PET scan in June 2010 (). In addition, follow-up with MRI has shown a clear reduction in contrast enhancement and a slight decrease in tumor size. Treatment was stopped in June 2010 since the disease was stable, and the patient was experiencing treatment-related fatigue. Since then the patient has been treatment free. The most recent methionine PET scan was carried out in October 2010, and did not show any signs of enhanced uptake in the tumor. MRI in August 2013 showed stable disease, and the patient now has no clinical symptoms of the tumor six years after diagnosis and 4½ years after introducing erlotinib and bevacizumab.
Figure 1.(A-D): 11C-methionine PET scans before and during chemotherapy. (A) In May 2009 after extensive radiotherapy, but before drug therapy, the scan showed enhanced uptake of methionine. (B) A treatment attempt with erlotinib in combination with cetuximab was made. Nevertheless, a slight increase in uptake was detected in July. (C) A clear reduction, even to subnormal levels, of methionine uptake in October compared to the previous exam was seen after replacement of cetuximab by bevacizumab, while erlotinib treatment continued. (D) The combination of erlotinib and bevacizumab continued until June 2010. The same month the methionine uptake level was unchanged compared to C.
Case 2
A female patient was 48 years old when diagnosed with a very large boneinfiltrating tumor mass in the sacrum in 2009. A laminectomy L4-S3 was performed in July 2009 and more extensive surgery in September 2009, with resection of approximately 80% of the extraosseous component. The decision to leave a residual tumor was based on the risk of causing neurological damage, in the light of tumor infiltration of spinal nerves. The histopathological diagnosis was consistent with chordoma. An MRI follow-up in September 2010 showed pronounced tumor progression. Clinical symptoms had also increased, with pain in the right gluteal region and radiating pain in the right leg. Re-operation was considered to be of no benefit, as the major part of the tumor component was intraosseous in the sacrum. The only surgical intervention deemed feasible was a sacrectomy, but this would obviously be associated with severe neurological sequelae. Thus, the patient was treated with conventional photon radiotherapy with 2 Gy fractions, to a total of 66 Gy, applied to a gross tumor volume (GTV) of 214 cm3. This regimen was given in November–December 2010. A PET/computed tomography (CT) with 18F-FLT, as marker for proliferation, done in April 2011 showed proliferative activity in the tumor, mainly in the residual intraosseous component (, white continuous arrows). The non-tumor affected bone marrow also showed high FLT uptake, which is a normal finding in non-irradiated bone marrow ( and B, white broken arrows).
Figure 2. (A and B): (A and B): 18F-FLT-PET/CT in April (A) and July (B) 2011. (A) FLT-uptake was high in the tumor, indicating enhanced proliferative activity (white continous arrows), before chemotherapy. The non-tumour affected bone marrow also showed high uptake (white broken arrows), but this is a normal finding. (B) In May erlotinib-bevacizumab was started, and in July no remaining FLT uptake could be seen in the tumor (white continous arrows). As expected, FLT-uptake in the non tumour affected bone marrow was still unchanged (white broken arrows).
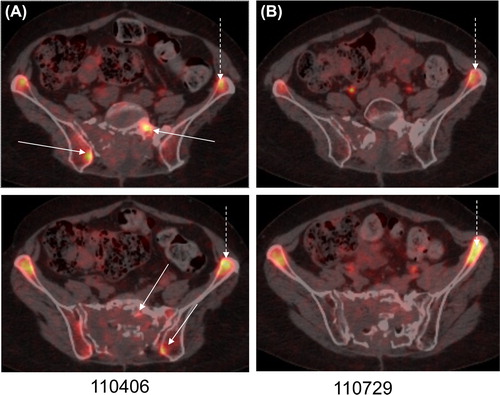
At this stage, May 2011, combination treatment with erlotinib and bevacizumab was initiated (erlotinib 150 mg once daily and bevacizumab 10 mg/kg once weekly), based on our previous experience [Citation8]. In July 2011, no remaining FLT-uptake remained in the tumor (), while unchanged uptake could be seen in the bone marrow. The patient was at this time suffering from paronychias and a superficial infection close to the left elbow. These conditions were considered to be therapy-related, and the treatment was stopped in October 2011. The wounds subsequently healed and so did the infection in the elbow after surgical wound revision. An FLT-PET/CT in September 2012 showed no increased proliferation, and MRI in August 2013 showed stable disease. The patient was at that time walking with the help of a walker, whereas she had been confined to a wheelchair before the treatment. The pain was well controlled with methadone and gabapentine. Rehabilitation was ongoing, with the aim of enabling the patient to return to part-time work as a school teacher.
Case 3
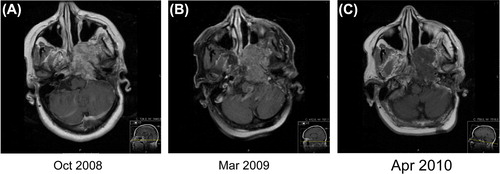
A woman was 43 years of age when she was operated in September 2005 with a transsphenoidal approach for a clivus chordoma, which had been confirmed by histopathology. Complementary re-operation was carried out in January 2007, with a presigmoideal, transsphenoidal approach. Postoperative recovery and rehabilitation were initially complicated by meningitis and the development of hydrocephalus. A ventriculoperitoneal shunt was therefore implanted in February 2007. An MRI in December 2007 showed a residual tumor of less than 1 cm in size. However, a follow-up MRI in October 2008 showed a marked increase in size to 5 × 5 × 5 cm (). As the patient was reluctant to undergo radiotherapy, treatment with erlotinib was started in December 2008 at a dose of 100 mg daily and cetuximab at 250 mg/m2. Cetuximab was stopped after only four cycles due to rather severe skin reactions, while erlotinib was maintained at 100 mg daily. A significant reduction in contrast enhancement in the tumor was seen at follow-up, from October 2008 (before erlotinib) (), compared to March 2009 () and April 2010 (), which was interpreted as secondary to inhibition of EGFR signaling. Regular MRI follow-up for more than two years showed stable disease. Unfortunately, two subsequent MRI examinations in March and August 2011 showed significant tumor progression with an additional tumor component of size 1 × 1.5 × 1 cm in the epipharynx, adjacent to the carotid vessels. Re-operation was discussed, but was considered too hazardous. The symptom that caused most distress to the patient at this time was pronounced neuralgic pain along the distribution of the left trigeminal nerve. Bevacizumab 10 mg/kg every second week was added to the erlotinib in September 2011. The patient experienced significant relief from the neuralgic pain almost immediately. Bevacizumab was stopped in November 2012 due to radiologically stable disease, while erlotinib was maintained. MRI in September 2013 showed stable disease. At the most recent visit at our department in October she was still in a good clinical status, showing no signs of neurological deterioration.
Figure 3. (A-C): Contrast enhanced T1 weighted MRI. (A) A contrast enhancing tumour with a size of 5×5×5 cm was observed in October 2008. (B) After treatment with cetuximab-erlotinib a clear decrease of contrast enhancement could be seen in March 2009. Due to toxicity cetuximab was stopped early, but treatment with erlotinib continued. (C) The contrast enhancement had decreased further in April 2010. The size of the tumour was unchanged.
Discussion
We describe here three cases of chordoma, one of them diagnosed on radiological and clinical grounds (Case 1), while two of them were histologically verified (Cases 2 and 3). These three patients demonstrated clinically meaningful responses to a combination of the VEGF inhibitor bevacizumab and the EGFR inhibitor erlotinib. Further surgical intervention was not a treatment option in any of the cases at this stage. Following initiation of the combination of erlotinib and bevacizumab, none of the patients in this report has neither experienced tumor progression nor clinical deterioration with follow-up times of 4½ years (Case 1), 2½ years (Case 2) and 2 years (Case 3).
The combination of erlotinib and bevacizumab in relapsing non-operable chordoma may lead to disease stability and increased quality of life with significant pain relief for years, at the cost of mild to moderate toxicity. The approach is promising and it is worthy of further investigation in a prospective controlled setting.
Conflict of interest: RH Member of the international steering committe of the Avaglio study evaluating bevacizumab (Roche®) for the treatment of gliobastoma.
TA National investigator of the Avaglio study and the Tamiga study (Roche®) for the treatment of glioblastoma.
References
- Eriksson B, Gunterberg B, Kindblom LG. Chordoma. A clinicopathologic and prognostic study of a Swedish national series. Acta Orthop Scand 1981;52:49–58.
- McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and survival patterns in the United States, 1973–1995. Cancer Cause Control 2001; 12:1–11.
- Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC, Jr. Lumbosacral chordoma. Prognostic factors and treatment. Spine 1999;24:1639–45.
- Zabel-du Bois A, Nikoghosyan A, Schwahofer A, Huber P, Schlegel W, Debus J, et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol 2010;97:408–12.
- Shalaby A, Presneau N, Ye H, Halai D, Berisha F, Idowu B, et al. The role of epidermal growth factor receptor in chordoma pathogenesis: A potential therapeutic target. J Pathol 2011;223:336–46.
- Dei Tos AP. Unveiling the molecular pathogenesis of chordoma: A new paradigm for molecular targeting of rare cancers. J Pathol 2011;223:565–6.
- Dewaele B, Maggiani F, Floris G, Ampe M, Vanspauwen V, Wozniak A, et al. Frequent activation of EGFR in advanced chordomas. Clin Sarcoma Res 2011;1:4.
- Asklund T, Danfors T, Henriksson R. PET response and tumor stabilization under erlotinib and bevacizumab treatment of an intracranial lesion non-invasively diagnosed as likely chordoma. Clin Neuropathol 2011;30:242–6.
- Launay SG, Chetaille B, Medina F, Perrot D, Nazarian S, Guiramand J, et al. Efficacy of epidermal growth factor receptor targeting in advanced chordoma: Case report and literature review. BMC Cancer 2011;11:423.
- Hof H, Welzel T, Debus J. Effectiveness of cetuximab/gefitinib in the therapy of a sacral chordoma. Onkologie 2006;29: 572–4.
- Linden O, Stenberg L, Kjellen E. Regression of cervical spinal cord compression in a patient with chordoma following treatment with cetuximab and gefitinib. Acta Oncol 2009; 48:158–9.
- Chen KW, Yang HL, Lu J, Wang GL, Ji YM, Wu GZ, et al. Expression of vascular endothelial growth factor and matrix metalloproteinase-9 in sacral chordoma. J Neurooncol 2011;101:357–63.
- Li X, Ji Z, Ma Y, Qiu X, Fan Q, Ma B. Expression of hypoxia-inducible factor-1alpha, vascular endothelial growth factor and matrix metalloproteinase-2 in sacral chordomas. Oncol Lett 2012;3:1268–74.
- Stacchiotti S, Longhi A, Ferraresi V, Grignani G, Comandone A, Stupp R, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol 2012;30:914–20.
