To the Editor,
Dental lesions related to anticancer drugs is a category of adverse effects often underestimated and overlooked, since they do not affect survival, although they may significantly affect the quality of life. Imatinib mesylate is an antineoplastic agent acting through inhibition of tyrosine kinases (TK), which are abnormally activated in certain malignancies. It is used as first-line treatment in chronic myeloid leukaemia (CML) [Citation1] and metastatic or locally aggressive gastrointestinal stromal tumours (GIST) [Citation2]. Its adverse effects, most common of which are oedema, muscle cramps, gastrointestinal symptoms (nausea, abdominal pain, diarrhoea), fatigue, rash, and musculoskeletal pains seldom necessitate cessation of therapy [Citation3]. Some of the drug's adverse reactions may be caused through “off-target” inhibition of tyrosine kinases in healthy tissues. Dental disease is not a recognised complication of imatinib treatment in humans [Citation3]. Herein, we report the first case of rampant dental disease associated with imatinib mesylate therapy in a patient with GIST. We postulate that dental lesions in patients treated with imatinib or similar TK inhibitors may reflect a direct effect of the drug on odontoblast development and function, resulting in dysregulation of tooth homoeostasis and defective dentine maintenance.
Case report
A 51-year-old, previously healthy, male presented with abdominal pain and difficulty in digesting his meals. Upper gastrointestinal endoscopy showed no abnormalities. Computerised tomograms (CT) and magnetic resonance imaging (MRI) of his abdomen revealed a 10 × 9.5 cm mass attached to the greater curvature of the stomach. Cytological examination of material obtained by fine-needle aspiration of the mass was compatible with GIST. The patient underwent abdominal gastrectomy, splenectomy, left adrenalectomy, and partial distal pancreatectomy. Histological examination of the completely excised tumour confirmed the diagnosis of CD117-positive GIST bearing a gain-of-function point mutation in the c-KIT gene. After the surgery, adjuvant chemotherapy was started with imatinib mesylate at 400 mg/day, which was well tolerated. Despite some initial loss of weight following gastrectomy, his nutritional state remained good. After the completion of two years of continuous chemotherapy, the patient began to complain that his teeth started to break easily. There was no periodontal disease. Almost one year after that, he has lost seven teeth and suffers from multiple lesions on the rest (). His dentist diagnosed dental erosion.
Figure 1. Expanded view of patient's teeth (top) and panoramic radiograph (bottom), showing multiple odontal coronary lesions with loss of hard tissue, missing teeth due to extractions following spontaneous fractures, and multiple dental restorations.
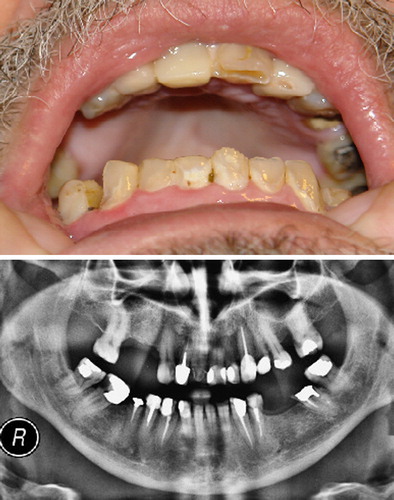
Results of haematological and biochemical investigations were unremarkable. In particular, serum calcium, phosphate, albumin and parathyroid hormone levels were normal. Serial CT and MRI studies of the abdomen showed no evidence of GIST recurrence. Imatinib was discontinued on completion of three years of treatment. The patient is currently undergoing extensive dental treatment including extractions, endodontic therapy, and placement of multiple restorations ().
Discussion
To our knowledge, this is the first report of dental disease associated with imatinib therapy in humans. It should be noted, however, that tooth lesions were among the long-term toxicities identified in a two-year toxicity study of imatinib in rats [Citation3]. Dental lesions including fractured incisors, dentine degeneration and necrosis, as well as necrosis of dental pulp and odontoblasts have also been described in rats receiving sunitinib, another multi-targeted TK inhibitor whose target profile overlaps with that of imatinib [Citation4].
Dental erosion – the loss of dental hard tissue by chemical processes that do not involve bacteria – may be caused by a multitude of extrinsic or intrinsic factors [Citation5,Citation6]. Extrinsic causes include diet (acidic drinks and foods), lifestyle (bad oral hygiene practices), environmental factors (inhalation of acid fumes), and medications. No dietary, environmental, or lifestyle factors could be identified in the case of our patient, whose dental health had been excellent before the onset of imatinib therapy. Intrinsic causes operate through gastric acid reaching the teeth as a result of vomiting or gastro-oesophageal reflux. Gastric acid could not have been the culprit in this gastrectomised patient.
Altered bone and mineral metabolism has been shown to occur in patients with either GIST or CML receiving imatinib. A significant percentage of them develop hypophosphataemia, hypocalcaemia and secondary hyperparathyroidism [Citation7]. Bone mineral density was recently shown to be decreased in 47% and increased in 2% of patients on long-term imatinib therapy [Citation8]. These alterations may be the result of the drug's effect on bone homoeostasis. The latter involves a mechanism of balanced bone resorption and new bone formation mediated through the activity of osteoclasts and osteoblasts, respectively. Imatinib inhibits a number of kinases (c-fms, c-kit, CAII, PDGFR, c-abl) that are known to participate in this process: M-CSF (the ligand for c-fms) and c-kit signalling is essential for osteoclast development and function; carbonic anhydrase II (CAII) participates in the dissolution of mineral from bone; platelet-derived growth factor receptor (PDGFR) signalling on osteoblasts modulates the production of osteoclastogenic M-CSF and RANKL [Citation9]. Moreover, osteoblast differentiation by bone morphogenetic protein (BMP)-2-induced signal transduction requires c-abl receptor tyrosine kinase activity [Citation10].
There is evidence that odontoblasts and odontoclasts act similarly to osteoblasts and osteoclasts producing and resorbing dentine, respectively. After tooth eruption, odontoblasts transform into a mature cell that survives throughout the life of the tooth. They constitute the majority of dental pulp cells and are involved in reparative dentine production in response to dental caries and other external injuries, transmission of sensory stimuli from the dentine, and in the cellular defense against bacteria [Citation11]. Odontoblasts express receptors for RANKL and M-CSF that are crucial for osteoclast and odontoclast formation [Citation12,Citation13]. Dental pulp cells also express c-kit, whereas BMPs (BMP2-BMP7) are expressed in cultures of dental pulp [Citation14,Citation15].
Our patient started to develop dental lesions after two years of imatinib therapy. By the end of his chemotherapy (three years) he suffers from severe loss of dental hard tissue that cannot be ascribed to any of the known extrinsic or intrinsic causes of dental erosion. In addition, his normal serum levels of calcium, phosphate, albumin and PTH indicate a cause different from mineral deficiency or malnutrition. It is tempting to postulate that imatinib has a direct effect on the odontoblast through inhibition of c-fms, c-kit, or the BMP signal transduction pathway. This would lead to impaired dentine maintenance and repair in response to microbial or other external insults. The incidence and exact molecular pathology of imatinib-induced dental damage should be the subject of future studies. We suggest that management of patients receiving imatinib on a long-term basis should include monitoring of dental health.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Soverini S, Martinelli G, Iacobucci I, Baccarani M. Imatinib mesylate for the treatment of chronic myeloid leukemia. Expert Rev Anticancer Ther 2008;8:853–64.
- Linch M, Claus J, Benson C. Update on imatinib for gastrointestinal stromal tumors: Duration of treatment. Onco Targets Ther 2013;6:1011–23.
- Gleevec (imatinib mesylate) prescribing information. [cited 2013 Oct 28]. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/gleevec_tabs.pdf).
- Patyna S, Arrigoni C, Terron A, Kim T-W, Heward JK, Vonderfecht SL, et al. Nonclinical safety evaluation of sunitinib: A potent inhibitor of VEGF, PDGF, KIT, FLT3, and RET receptors. Toxicol Pathol 2008;36:905–16.
- Zero DT. Etiology of dental erosion – extrinsic factors. Eur J Oral Sci 1996;104:162–77.
- Scheutzel P. Etiology of dental erosion – intrinsic factors. Eur J Oral Sci 1996;104:178–90.
- Berman E, Nicolaides M, Maki R, Fleisher M, Chanel S, Scheu K, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med 2006;354:2006–13.
- Berman E, Girotra M, Cheng C, Chanel S, Maki R, Shelat M, et al. Effect of long term imatinib on bone in adults with chronic myelogenous leukemia and gastrointestinal stromal tumors. Leuk Res 2013;37:790–4.
- Vandyke K, Fitter S, Dewar AL, Hughes TP, Zannettino ACW. Dysregulation of bone remodeling by imatinib mesylate. Blood 2010;115:766–74.
- Ghosh-Choudhury N, Mandal CC, Das F, Ganapathy S, Ahuja S, Ghosh-Choudhury G. c-Abl-dependent molecular circuitry involving Smad5 and phosphatidylinositol 3-kinase regulates bone morphogenetic protein-2-induced osteogenesis. J Biol Chem 2013;288:24503–17.
- Couve E, Osorio R, Schmachtenberg O. The amazing odontoblast: Activity, autophagy, and aging. J Dent Res 2013; 92:765–72.
- Rani CS, MacDougall M. Dental cells express factors that regulate bone resorption. Mol Cell Biol Res Commun 2000;3:145–52.
- Duan X, Yang T, Zhang Y, Wen X, Xue Y, Zhou M. Odontoblast-like MDPC-23 cells function as odontoclasts with RANKL/M-CSF induction. Arch Oral Biol 2013; 58:272–8.
- Gagari E, Rand MK, Tayari L, Vastardis H, Sharma P, Hauschka PV, et al. Expression of stem cell factor and its receptor, c-kit, in human oral mesenchymal cells. Eur J Oral Sci 2006;114:409–15.
- Rodriguez-Lozano FJ, Insausti CL, Iniesta F, Blanquer M, Ramirez MC, Meseguer L, et al. Mesenchymal dental stem cells in regenerative dentistry. Med Oral Patol Oral Cir Bucal 2012;17:e1062–7.
