Abstract
Background. The simultaneous presence of cancer and other medical conditions (comorbidity) is frequent. Cigarette smoking is the major risk factor for as well head and neck cancer (HNC) and lung cancer (LC) as chronic obstructive pulmonary disease (COPD). COPD is the most common comorbidity in LC patients, and presence of COPD worsens the prognosis of HNC and LC. COPD is under-diagnosed and under-treated in the Danish population. The aims of this study were to determine the prevalence of COPD in a HNC and LC population, and to determine the need and feasibility of a randomized controlled phase II trial comparing usual care with optimized medical treatment of COPD in cancer patients.
Material and methods. All patients with HNC or LC referred for oncologic treatment in a university hospital during a 10-month period were invited to attend a pulmonary clinic for evaluation of lung function. Patients who were found to have concomitant COPD were randomized to intervention or usual care. Primary endpoints were prevalence of COPD among the referred patients with either LC or HNC, and further whether the patients that were diagnosed with COPD already received treatment in accordance with Danish COPD guidelines. Secondary outcome was feasibility, i.e. the proportion of eligible patients that accepted follow-up in the pulmonary clinic for 24 weeks in addition to oncological treatment. The design of the randomized trail is described in detail.
Results. In total 130 patients of whom 65% had LC and 35% HNC have been screened during the first seven months of this ongoing trial. Sixty-eight percent of LC patients and 22% of HNC patients had COPD. All but one of 68 eligible patients accepted randomization. Nearly one third (31%) of the LC and HNC patients with COPD were diagnosed prior to study entry, and of these, only 33% were receiving correct treatment according to current guidelines.
Conclusion. For patients with LC, and to a lesser extend HNC, there is a need for improved diagnosis and treatment of concomitant COPD. Furthermore, patients found it acceptable to be scheduled for a 24-week follow-up in a pulmonary clinic along with their oncological treatment.
Lung cancer (LC) causes more deaths than breast-, colon- and prostate cancer combined. In Denmark, the annual number of new cases is 4400, with the majority of cases diagnosed at advanced stages, and 3700 die from LC annually [Citation1]. Head and neck cancer (HNC) is a less frequent cancer with an annual number of new cases and deaths of 1000 and 300, respectively [Citation1]. Chronic obstructive pulmonary disease (COPD) is widely under-diagnosed and under-treated but nevertheless expected to become the third leading cause of death and disability worldwide by in 2020 [Citation2,Citation3]. It is estimated that there are between 300 000 and 400 000 COPD patients in Denmark, of which only 100 000 are receiving medical treatment [Citation4]. A recent study from Scotland estimated that 43% of a LC population suffered from synchronous COPD, thereby being the most prevalent comorbidity [Citation5]. Furthermore, recent data from LC screening trials suggest that COPD is associated with more aggressive LC compared with LC found in non-COPD smokers [Citation6].
Several studies have shown a correlation between comorbidity, including COPD, and survival of HNC and LC [Citation7,Citation8]. These studies combined data from several disease registries, where information on comorbidity was derived from hospital discharge- and outpatient registers. Comorbidity was grouped using the Charlson Comorbidity Index, which counts the number of comorbid conditions but not severity. Although these studies are able to include very large number of patients, the disadvantage of this method is that it examines the medical history of the individual rather than actual comorbidity burden at time of diagnosis. There is very limited knowledge about possible effects of correctly treated concomitant COPD in patients with smoking-related cancers. The presence of COPD has in some studies (but not all) been shown to worsen the prognosis of non-small cell LC independently [Citation9–11]. Regardless of intention of treatment (curative, palliative) proper management of comorbidity is likely to improve patient quality of life (QoL) and survival. Therefore the objectives of the present study were to determine the prevalence of coexisting COPD in patients with newly diagnosed HNC and LC, and the proportion of COPD patients who were – at time of entry – being treated in accordance with Danish COPD guidelines. As an indicator of the feasibility of operating an out-patient pulmonary clinic within the department of oncology, the proportion of eligible patients that accepted a 24-week follow-up in the pulmonary clinic as part of a randomized study was determined.
Material and methods
The COPD management in LC and HNC study is a randomized phase II study testing the hypothesis that COPD follow-up on a regular basis with active intervention by medication can improve the PS and QoL. All newly diagnosed HNC and LC patients referred to the Department of Oncology, Herlev Hospital were screened for COPD and if present, invited to participate in a randomized trial. The study has three components: screening for COPD among newly diagnosed HNC and LC patients, determining whether the described set-up is feasible, and a randomized intervention for patients with COPD. The screening part of the study provides information on prevalence of COPD, severity by global obstructive lung disease (GOLD) stage and whether the patients receive adequate medication according to guidelines. The randomized part of the study will assign patients to either continuous follow-up with pulmonologist or usual management.
The randomized study was designed as a non-blinded controlled trial. A COPD outpatient clinic was established in February 2014 at the Unit of Thoracic Oncology and was staffed by pulmonologists. The inclusion is planned to end in December 2014. Patients diagnosed with either LC or HNC and COPD were randomized to either control or intervention. Patients in the control group were to continue their current, if any, treatment of COPD without any adjustments. Patients in the intervention group were offered treatment of COPD according to GOLD guidelines, and were scheduled for two control appointments at 12 and 24 weeks where adjustment of the COPD treatment was considered.
Measures
At first visit, the patients filled out QoL questionnaires: EORTC (Q30 + LC13) and CAT-score [Citation12,Citation13]. EORTC is a validated QoL questionnaire for cancer patients. CAT is an eight-question questionnaire covering the most frequent symptoms of COPD and validated to determine disease severity. At 13 weeks and 25 weeks CAT and EORTC questionnaires were sent to both intervention- and control patients.
Participants
All newly diagnosed patients with LC and HNC referred to the Department of Oncology at Herlev Hospital were screened for COPD. The only patients not entering the screening phase of the study were those who were planned to have no or short duration of treatment (less than one month). Patients were seen at the pulmonary clinic within the first two weeks after referral. The LC patients include all primary LCs, and the HNC patients include primary cancers in larynx, hypopharynx, oral cavity, pharynx and oropharynx. Patients treated with curative as well as palliative intent were eligible. At the first visit in the pulmonary clinic, spirometry was performed, regardless of a prior COPD diagnosis. Reversibility testing was done unless the patient had used a beta-2-agonist the same day, in order to exclude patients with asthma. Patients with a FEV1/FVC ratio < 0.70 and no significant beta-2-reversibility were eligible for the randomized trial, and were invited.
Randomization
Randomization was stratified to ensure equal distribution of sex, age, LC and HNC between the control group and the intervention group. As primary endpoint for the power calculation CAT score was used. It has been estimated that the minimal clinical important difference (MCID) for CAT with a relevant intervention is -2 points [Citation14]. Hence, to detect a statistical significant difference with a power of 0.80 and a significance level (α) of 0.05 in a two-sided test, a total sample size of 64 individuals was needed (32 in each arm).
Inclusion
Eligible patients were randomized after written consent. After randomization, medical history and baseline information was obtained. Based on spirometry, exacerbation history, MRC-grade and CAT-score, the GOLD group was determined () (15).
Intervention group
The pulmonary physician assessed whether the current COPD medication was in accordance with the GOLD guidelines, and initiated treatment in those patients diagnosed at study entry. Choice of drug and device was done in dialogue with the patients. Patients were asked to demonstrate inhalation technique using a placebo device to make sure that the medication was taken correctly. At the follow-up visits, any need for changes in the COPD medication was assessed by the physician and discussed with the patient. Also, inhalation technique was checked again. If patients did not show up for a scheduled visit, the physician would call the patient and arrange for a new consultation. Patients were instructed to book extra visits or telephone consultations between planed appointments with the pulmonologist if needed.
Control group
For the patients in the control group, no changes in the medication were suggested, but generally the patients were encouraged to see who ever they are already seeing for respiratory issues. All patients were informed of the COPD diagnosis found at screening.
Follow-up
The follow-up period was 25 weeks. CAT and EORTC questionnaires were sent to both patients in the intervention group and control group at week 13 and 25. The patients in the intervention group thus received the questionnaires approximately 10 days after the visits to the pulmonary clinic. Should the questionnaires not be returned within one week, the pulmonary physician would remind the patient by phone. If the questionnaires were not returned after four weeks, the patient was considered to be lost to follow-up with respect to QoL but was followed up for survival using population registers. The last control visit is expected to be in June 2015.
Outcomes
Endpoints of the randomized trial were QoL (EORTC and CAT) and overall survival. These data will be available at a later stage.
Statistical methods
SPSS 21 was used to analyze data (IBM Corp. Armonk, NY). Differences in baseline data were analyzed with χ2-test. Feasibility was assessed as the proportion of screened eligible patients that accept inclusion and randomization.
The secondary outcomes will be analyzed at a later stage. Feasibility of the study will be evaluated as percentage of eligible patients who complete the study, and OS will be evaluated in all patients enrolled (ITT population) using Kaplan-Meier plots and log rank tests.
Results
The results reported in this paper are the preliminary screening data and preliminary data on feasibility of the intervention.
shows a consort diagram for patients who have been screened in the first seven months. A total of 130 patients were screened in the pulmonary clinic, and 67 patients were found to be eligible for randomization (52%). In , patient characteristics for the participants so far enrolled in the randomized trial are shown. Mean age was 68 years, the majority of patients were male (63%), and had LC (87%). COPD was the most prevalent comorbidity followed by cerebrovascular and heart disease. Most of the patients were current smokers (75%) and the mean number of pack-years was high (44 years). Spirometry findings by cancer type are shown in . The prevalence of COPD was higher among patients with LC (68.2%) than patients with HNC (22.2%), p < 0.05. Two LC patients were not able to perform acceptable spirometry due to cerebral impairment. One HNC patient could not perform spirometry due to tracheostomy.
Figure 2. Preliminary inclusion and exclusion in the randomized controlled trial “COPD management in lung and head-and-neck cancer”.
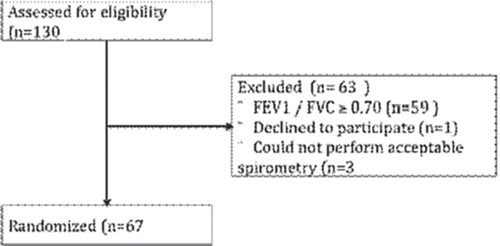
Table I. Preliminary sample of participants in a randomized controlled trial “COPD management in lung and head-and-neck cancer”.
Table II. Spirometry findings by cancer type.
All but one patient diagnosed with COPD accepted inclusion in the randomized study. The one eligible patient that refused participation was a HNC patient that had clear clinical signs of COPD and a FEV1 < 40% of expected. He gave no reason.
shows the result of the COPD classification according to GOLD guidelines, and whether patients were diagnosed with COPD before the first visit. Only 21 (31.3%) were previously diagnosed with COPD, and only seven of these patients received proper treatment according to guidelines. In Gold group D, 12 (54.5%) patients were diagnosed, but only three of these patients were treated according to guidelines. For patients with mild symptoms and low risk in Gold group A, no inhalation treatment is in accordance with guidelines. Thus all patients in this group were classified as receiving correct treatment.
Table III. Number of patients previously diagnosed and previously treated according to guidelines, by GOLD classification.
Discussion
A substantial number of LC and HNC patients were not previously diagnosed with COPD, and only a limited number received proper treatment according to guidelines. All but one patient accepted inclusion and randomization in this trial. Based on these preliminary findings, the study design appears to be acceptable to the patients, and able to identify a substantial number of undiagnosed or under-treated COPD patients. Whether the intervention will positively affect the outcome for patients must await the results of the randomized trial. The findings regarding under-diagnosis of COPD are in line with previous findings in the general Danish population, where a large part of COPD patients are not diagnosed and thus not treated according to guidelines [Citation4]. It is perhaps surprising that COPD is not diagnosed in this patient group as they were all smokers or former smokers, and very recently underwent diagnostic work-up. It is possible that when a cancer diagnosis is made, other medical conditions are considered of less importance. This would actually lead to cancer patients having poor management of comorbid conditions that potentially would have a detrimental effect on outcomes, such as QoL and survival. The design of this study may not be optimal for HNC patients because COPD is not as frequent as in LC. Furthermore, HNC patients do in some cases have difficulties performing the spirometry due to cancer location, and often go through intense treatment programs with daily radiotherapy which leaves little time for extra visits to the clinic.
Dyspnea, phlegm and cough can be caused both by cancer and COPD, and the same symptoms are frequent adverse events to anticancer treatment. In the palliative setting, comorbidity has been found to be a negative prognosticator when analyzed univariably, but was found to be less important than performance status in a multivariate analysis of Danish LC patients [Citation8]. However, if the performance status and ability to cope with chemotherapy is partly driven by COPD symptomatology, an active intervention could lead to better tolerance to cancer therapy and increased QoL.
An important part of optimal COPD treatment is pulmonary rehabilitation with exercise and education. It could be beneficial in the future to incorporate COPD rehabilitation in the oncology/pulmonary clinic, and perhaps to broaden the scope to general comorbidity management with the ability to screen and treat other frequent smoking related diseases, e.g. cardiovascular diseases (16).
Conclusion
This study suggests that screening for, and treatment of COPD in LC patients is feasible within an oncological department, and confirms that the disease is under-diagnosed and under-treated. For HNC patients, the COPD frequency is much lower than in LC and the present study design, although acceptable, might thus not be optimal in HNC patients.
Acknowledgments
The study was supported by an unrestricted grant from Boehringer Ingelheim Denmark.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase. International Agency for Research on Cancer; 2013. [cited 2014 Sep 20].
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65.
- Mannino DM, Buist AS. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007;370: 765–73.
- Hansen JG, Pedersen L, Overvad K, Omland Ø, Jensen HK, Sørensen HT. The Prevalence of chronic obstructive pulmonary disease among Danes aged 45–84 years: Population-based study. COPD 2008;6:347–52.
- Grose D, Morrison DS, Devereux G, Jones R, Sharma D, Selby C, et al. Comorbidities in lung cancer: Prevalence, severity and links with socioeconomic status and treatment. Postgrad Med J 2014;90:305–10.
- Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380–86.
- Luchtenborg M, Jakobsen E, Krasnik M, Linklater KM, Mellemgaard A, Moller H. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur J Cancer 2012;48:3386–95.
- Boje CR, Dalton SO, Gronborg TK, Primdahl H, Kristensen CA, Andersen E, et al. The impact of comorbidity on outcome in 12 623 Danish head and neck cancer patients: A population based study from the DAHANCA database. Acta Oncol 2013;52:285–93.
- Asmis TR, Ding K, Seymour L, Shepherd FA, Leigh NB, Winton TL, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: A review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 2008;26:54–9.
- Putila J, Guo NL. Combining COPD with clinical, pathological and demographic information refines prognosis and treatment response prediction of non-small cell lung cancer. PLoS One 2014;9:e100994.
- Lee SJ, Lee J, Park YS, Lee CH, Lee SM, Yim JJ, et al. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol 2014;9:812–7.
- COPD Assessment Test. [cited 2014 Sep 20]. Available from: http://www.catestonline.org/.
- Fayersa P, Bottomleyc A. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer 2002;38: 125–33.
- Kon SS, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, et al. Minimum clinically important difference for the COPD Assessment Test: A prospective analysis. Lancet Respir Med 2014;2:195–203.
- Global Strategy for Diagnosis, Management and Prevention of COPD 2014, © Global Initiative for Chronic Obstructive Lung Disease (GOLD), all rights reserved. Available from http://www.goldcopd.org/
- Cavaillès A, Brinchault-Rabin G, Dixmier A, Goupil F, Gut-Gobert C, Marchand-Adam S, et al. Comorbidities of COPD. Eur Respir Rev 2013;22:454–75. Accessed on 15th November, 2014.