Abstract
Background. T-cell lymphoblastic lymphoma (T-LBL) is a rare neoplasm of precursor lymphoblast origin, for which there is no standard treatment for adults. Results of current treatment strategies in selected populations do exist but are largely unreported for unselected series. Here, we aimed to investigate treatment outcome in a population-based cohort.
Material and methods. Patients were identified through the Swedish Lymphoma Registry and data was retrospectively collected for all adult (≥ 18 years) Swedish T-LBL patients diagnosed during 2000–2009.
Results. A total of 39 patients with median age 40 years (range 18–78) were identified with females being significantly older than males (median age 66 vs. 37, p = 0.027). The five-year overall survival for all patients was 42%. Female gender was associated with shorter survival also when adjusted for treatment strategy and age [hazard ratio (HR) 4.29; p = 0.002]. Thirty patients received intensive chemotherapy, otherwise used for treatment of acute lymphoblastic leukemia (ALL), which resulted in an overall response rate of 97% and a five-year progression-free survival (PFS) of 49%. In this group only CNS involvement at diagnosis predicted shorter PFS (HR 13.3; p = 0.03). Among patients treated with hyper-CVAD the addition of mediastinal irradiation resulted in prolonged time to progression compared to patients receiving only chemotherapy (p = 0.047). The major reason for treatment failure was relapse and in this series 18-fluoro-deoxyglucose positron emission tomography (PET) did not predict this risk.
Conclusion. This population-based study indicates that all fit T-LBL patients should be considered for intensive treatment. Our results also suggest a beneficial effect of mediastinal irradiation in combination with hyper-CVAD treatment. Relapsing patients have a dismal outcome irrespective of salvage treatment.
T-cell lymphoblastic lymphoma (T-LBL) is a rare disease of precursor T-cell origin representing a lymphoma variant of T-cell acute lymphoblastic leukemia (T-ALL). T-LBL is most common in children and young adults with a male preponderance and it typically presents with a large mass in the anterior mediastinum. Pleural and pericardial effusion is common and the disease has a high risk of central nervous system (CNS) involvement. Morphologically and immunophenotypically T-LBL and T-ALL are very similar and classified as one entity in the WHO classification. The distinction of T-LBL from T-ALL is usually made with respect to the degree of bone marrow involvement, naming cases T-LBL if there is 25% or less infiltration [Citation1]. As for T-ALL, deregulation of NOTCH1 signaling in many cases seem to be important for the evolution of T-LBL [Citation2] but at gene expression level there are indications of differences between T-LBL and T-ALL [Citation3,Citation4].
Initial therapeutic strategies based on CHOP-like chemotherapy yielded poor long-term survival [Citation5]. Following reports of improved results in children treated with intensive ALL-type chemotherapy [Citation6] this strategy has been adopted also for the treatment of adults. Due to the rarity of the disease there are few prospective trials specific for T-LBL in adults and most data originates from retrospective reports on the specific outcome of T-LBL patients enrolled in large LBL/ALL studies.
ALL-type treatment typically consists of an induction treatment followed by a consolidation phase with re-inductions. Maintenance treatment with chemotherapy for up to two years is part of the consolidation in some protocols. High-dose chemotherapy and autologous stem cell transplantation (SCT) instead of maintenance chemotherapy has been reported to improve survival in a retrospective study [Citation7] but a prospective trial resulted in a similar outcome between the two strategies [Citation8]. In a retrospective investigation, allogeneic stem cell transplantation was not associated with a clear benefit over autologous SCT with regard to long-term survival [Citation9]. The role of mediastinal irradiation has also been investigated without conclusive results [Citation10,Citation11]. With ALL-type treatment long-term survival between 50% and 70% has been reported [Citation10,Citation12,Citation13] but no standard treatment strategy has been established. The major concern with current treatment strategies is relapse, since recurrent disease has a very poor prognosis [Citation14]. Unfortunately, risk factors for relapse after ALL-type treatment have been hard to establish.
To our knowledge, there are no reports on the outcome for adult T-LBL, with T-ALL excluded, in an unselected population using current treatment strategies. We therefore aimed to investigate the outcome in a Swedish population-based cohort.
Material and methods
The Swedish Cancer Registry (SCR) is a national registry to which pathologists and clinicians are obliged to report every case of malignancy diagnosed. However, at the level of specific lymphoma classification the SCR contains limited information. Due to this the Swedish Lymphoma Group in January 2000 launched the Swedish Lymphoma Registry (SLR) containing more detailed information, covering all lymphoma patients from the age of 18 years. When the SCR receives a lymphoma diagnosis, it notifies the Regional Cancer Center that sends the SLR form to be completed by the clinician responsible for the patient. In 2007 information contained in the SLR was extended to include information on treatment and response. Since the start of the SLR the coverage has been at the level of 95–97% compared to the SCR.
Study population
All Swedish patients diagnosed with T-LBL between 1 January 2000 and 31 December 2009 were identified through the SLR. In total, 46 patients were initially registered in the SLR as having a diagnosis of T-LBL during this time period. However seven patients had an infiltration > 25% of bone marrow cellularity and were re-classified as T-ALL and excluded from this study. The diagnosis of T-LBL was established in routine clinical care by histology and immunohistochemistry and followed the 2001 edition of the WHO classification of lymphoid neoplasms [Citation1]. Basic clinical data was collected from the SLR and after informed consent further data was collected retrospectively from the individual patient records. Of the remaining 39 patients one individual declined further participation. One patient's record could not be retrieved and thus, only basic data from the registry was available. For surviving patients the median follow-up was 6.5 years.
Cerebrospinal fluid (CSF) cytology was examined in all patients in the intensive treatment group. Evaluation of treatment response included computed tomography (CT) scanning and bone marrow examination for the patients in the intensive treatment group. PET scan was included in the post-induction evaluation at the discretion of the treating physician. These examinations were performed at variable time points from the start of treatment but all patients were evaluated before the start of consolidation treatment. The present study was approved by the Regional Ethical Board, Lund, Sweden.
Statistics
Treatment response was classified according to the International Harmonization Criteria [Citation15]. OS was defined as time from diagnosis to death or last follow-up. PFS was defined as time from diagnosis to relapse/progression or death from any cause. Time to progression was defined as time from diagnosis to relapse/progression or lymphoma-specific death. All analyses were made on an intention to treat basis. Distribution differences of clinical characteristics between groups were analyzed with χ2-test and age differences with Mann-Whitney U-test. Survival curves were estimated with the Kaplan-Meier method, groups were compared using log rank test and risk factor analysis was made using Cox proportional hazard ratios. Factors were analyzed in univariable analysis and all factors with p ≤ 0.1 were retained in the multivariable analysis. All p-values were two sided and values were regarded statistically significant if p ≤ 0.05. All statistics were performed with SPSS version 19.
Results
Patient characteristics
The median age was 40 years (range 18–78) with a male:female ratio of 1.6:1. Females were older than males (median age 66 vs. 37 years, p = 0.027). Clinical characteristics at diagnosis are listed in . Almost half of the patients presented with stage IV disease either with bone marrow infiltration or extensive involvement of one or more extra- lymphatic organs. Two patients presented with a vena cava superior syndrome and two patients had CNS involvement at diagnosis. Patients older than 60 years had significantly less often bulky disease (> 10 cm) compared to younger patients (36% vs. 82%, p = 0.007) as well as less often pericardial and pleural effusions (p = 0.032, respectively, p = 0.008). One patient had a prior diagnosis of hematologic malignancy (indolent B cell lymphoma). One case with negative staining for terminal deoxynucleotidyl transferase (TdT) was included. This patient displayed histological, immunophenotypical and clinical characteristics that in all other aspects were typical of T-LBL. One patient was not tested for TdT-staining and the diagnosis of T-LBL was established based on immunophenotypic and histologic findings. The remaining patients all had TdT-positive lymphomas.
Table I. Clinical characteristics at diagnosis for the entire cohort (N = 39).
Treatment
All patients received chemotherapy and the choice of treatment was made on an individual basis. For analytical purpose regimens were grouped into intensive or non-intensive regimens as listed in . Patients treated with non-intensive regimens (median 74 years, range 55–77) were older (p < 0.001) compared to patients receiving intensive treatment (median 37 years, range 18–66), but had a similar WHO performance status.
Table II. Induction treatment.
Patients received an array of ALL-type induction treatments as listed in . The choice of regimen was to some extent center-related and patients receiving LSA2L2 induction were older compared to patients treated with other induction regimens (p = 0.002). Details for the various regimens have been described earlier [Citation16–21], except for the VSTB-95 regimen. This very intensive protocol was developed for the treatment of pediatric lymphoma patients by VSTB (Swedish working group for the treatment of solid tumors in children) and consists of an induction phase, re-induction, three CNS oriented blocks and a late re-induction maintenance followed by maintenance therapy with 6-mercaptopurine and oral methotrexate.
For the two patients with CNS involvement at diagnosis treatment consisted of hyper-CVAD with alternate intrathecal injections of methotrexate and cytarabine twice weekly until disease clearance from the CSF after which they received additional intrathecal methotrexate prophylaxis but no CNS irradiation. All other intensively treated patients received intrathecal prophylaxis but no CNS irradiation.
Mediastinal irradiation was given on an individual basis at the discretion of the physician, with none of the induction treatments precluding this option. Four patients, all treated with hyper-CVAD, had mediastinal irradiation as part of their primary treatment. One patient presenting with a superior vena cava syndrome received immediate radiation therapy at a dose of 21 Gy before chemotherapy was initiated while three patients received irradiation, at doses between 30 and 36 Gy, after induction chemotherapy.
Consolidation treatment was given to 25 of the 30 intensively treated patients as listed in . Reasons for not receiving consolidation were toxicity during induction treatment in three patients, early relapse in one patient and unclear in one case. Four patients were treated with SCT after induction treatment (two autologous SCT and two allogeneic SCT) and the remaining 21 patients had maintenance chemotherapy for up to two years.
Table III. Maintenance and consolidation therapy.
Adverse events and treatment-related deaths
In the group of patients treated with non-intensive regimens (n = 7), three patients died during treatment; one from septicemia, one by unknown cause shortly after the first chemotherapy cycle and one patient died from pulmonary aspergillosis. The remaining patients in this group had no major complications to treatment.
No treatment-related deaths occurred during induction treatment in the intensive treatment group. Febrile neutropenia was common, resulting in minor treatment delays. Fourteen of 19 patients (74%) treated with hyper-CVAD received the planned number of treatments without major complications. Two patients treated with pediatric protocols (VSTB-95 and NOPHO-ALL-92) were switched to less intensive second-line therapy and maintenance respectively due to excess toxicity.
Treatment outcome
Evaluation of treatment response was performed at variable time point before the start of consolidation treatment. Thirteen patients were evaluated with the addition of PET but no pre-treatment PET had been performed in any of these patients.
Evaluation of treatment response was possible in 35 of 39 patients. The overall response rate (ORR) for the cohort was 30/35 (85%) with 12/35 (34%) achieving complete remission (CR) and 18/35 (51%) partial remission (PR). Among non-intensively treated patients none of the evaluable cases reached a CR. In the intensively treated group ORR was 97% with 57% CR and 40% PR, () and in this group only one patient in PR was switched to salvage treatment. The remainder of PR's consisted of small residual masses and, with the exception of two patients receiving mediastinal irradiation, did not influence therapy decisions. For all patients evaluated with PET after induction treatment the examination was assessed as normal.
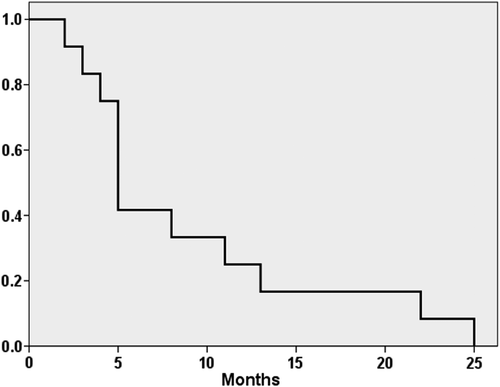
In the entire series 22 patients died and among intensively treated patients 15 of 30 patients died. In the latter group 12 patients experienced relapse, two patients developed secondary hematologic malignancies (one myelodysplastic syndrome and one pre-B-ALL), one patient died from complications to allogeneic SCT in first complete remission. This resulted in an estimated five-year PFS and OS of 42% for the entire cohort (). For intensively treated patients the calculated five-year PFS and OS was 49% and 48%, respectively, as shown in and C. Despite normal PET-CT at evaluation seven of 13 patients (54%) relapsed. CNS and mediastinum were the most common sites of relapse (see details in ). Three patients experienced isolated CNS relapse, including one patient with CNS involvement at diagnosis, while another two had CNS relapse as part of a disseminated disease recurrence. All relapses occurred within 27 months from diagnosis with six relapses during ongoing maintenance chemotherapy. There were no statistically significant associations between type of induction treatment or number of intrathecal injections and CNS-relapse (data not shown).
Figure 1. Kaplan-Meier estimates of overall (OS) and progression-free survival (PFS) among T-cell lymphoblastic lymphoma patients. (A) OS (solid line) and PFS (dashed line) for the entire cohort. (B) OS, intensive treatment group (solid line) and non-intensive group (dashed line). (C) PFS, intensive treatment group (solid line) and non-intensive group (dashed line).
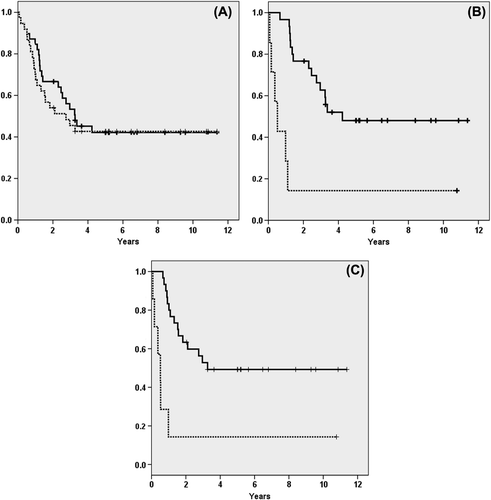
Table IV. Relapse treatment and site of relapse.
Among patients treated with hyper-CVAD the addition of mediastinal irradiation (n = 4) resulted in a longer time to progression (p = 0.047, log-rank test) compared to patients that received only hyper-CVAD (n = 15). In the former group none of the patients experienced a relapse compared to nine in the latter.
A wide range of salvage treatments were used and are listed in . Three patients proceeded to allogeneic SCT and two patients underwent an autologous SCT as part of the relapse treatment. Except for one patient who developed fatal complications after allogeneic SCT, all other relapsed patients eventually died from progressive lymphoma ().
Prognostic factors
All clinical characteristics at diagnosis, listed in , as well as intensive/non-intensive treatment were analyzed as predictors for OS and PFS (data not shown). In the entire cohort, age, female gender and non-intensive treatment were significant adverse factors for OS and PFS in univariable analysis (see ). In a multivariable analysis non-intensive treatment and female gender retained significance for a shorter OS while female gender was the only factor of significance for shorter PFS.
Table V. Risk factor analysis for overall survival (OS) and progression-free survival (PFS).
In the intensive treatment group age was not predictive for OS (HR = 0.998; p = 0.930) or PFS (HR = 0.997; p = 0.856). Three of the four patients over 60 years of age who received intensive treatment are still alive in continuous remission. Only CNS disease at diagnosis showed statistically significance in predicting a shorter OS (HR = 7.44; p = 0.017) and PFS (HR = 13.3; p = 0.005) in univariable analysis. In multivariable analysis CNS disease did not reach the level of significance for prediction of shorter OS but remained significant as a risk factor for shorter PFS (HR = 8.96; p = 0.030) ().
Discussion
The outcome of adult T-LBL patients treated with ALL-type chemotherapy doubtlessly compares favorably to historical results with CHOP-based treatment. Reports of this strategy are mostly limited to selected patient populations from clinical trials or populations selected in other ways [Citation22]. Population-based materials on adult T-LBL are scarce in the literature [Citation23] and there is to our knowledge no published data focusing specifically on T-LBL outcome in a completely unselected population, treated according to current strategies.
Here we report the results for all Swedish adult T-LBL patients during a 10-year period, from 2000 to 2009. Without national guidelines for the treatment of adult T-LBL patients many different chemotherapy regimens were used. This fact, in combination with the low number of patients, are major limitations of the present study and make comparisons between intensive treatment strategies difficult. Another limitation of this study is the lack of central pathology review and we have included one patient with negative TdT-staining as this has been described previously [Citation24]. The clinical characteristics of the patients in our material largely fit into previous descriptions [Citation1]. The median age in our cohort is higher than in clinical trials since T-LBL, although very rarely, still occurs among elderly patients who may not be included in series with uniform treatment. As expected and well described before, there was a male predominance among the patients. Somewhat surprisingly we found females to be significantly older than males and older patients (age ≥ 60 years) had less often bulky disease, pleural and pericardial effusions. It cannot be excluded that this reflects true clinical differences related to age since the age cut-off in clinical trials eliminates the possibility to detect such characteristics.
Long-term survival in LBL in recent reports from clinical trials has varied between 51% and 72% [Citation7,Citation10,Citation12,Citation13]. Since our cohort included patients that received CHOP-like treatment the five-year OS of 42% of the whole cohort is inferior to these results. Non-intensive treatment was one of the factors that predicted a shorter overall survival in multivariable analysis while age and classical lymphoma risk factors, e.g. IPI, did not predict outcome in our study. The other factor associated with a shorter overall survival in multivariable analysis was female gender. The inferior outcome among females was not expected, and can only in part be explained by the fact that there was a female dominance in the group that received non-intensive treatment. Among intensively treated patients there was no significant difference in outcome between genders, but unfortunately the group of non-intensively treated patients was too small for a multivariable risk factor analysis.
In our material there were 30 patients known to receive ALL-type treatment and also in this group the estimated five-year OS of 48% is inferior to what has been reported from clinical trials, possibly explained by the population-based nature of our cohort including patients with different comorbidities.
There were several complications to treatments but the overwhelming problem was relapsing disease. As reported earlier, prognosis after relapse was extremely poor [Citation14]. In our series none of 12 relapsed patients survived, 11 whom died from progressive disease, despite three of them undergoing allogeneic SCT in second remission. Factors that predict the risk of relapse after ALL-type treatment have been hard to establish and not consistent between studies [Citation7,Citation10,Citation12]. In our material only CNS-involvement at diagnosis predicted a shorter PFS. This must be cautiously interpreted, since there were only two patients with CNS-involvement in our series. However, both patients in our study were treated with hyper-CVAD and our finding is the same as in the study by Thomas et al. [Citation13], where CNS-involvement at diagnosis was the only predictor for a shorter PFS among T/B-LBL patients treated with this regimen. Our results, in accordance with the results by Thomas et al., suggest that hyper-CVAD without cranial irradiation might not be a sufficient treatment for patients presenting with CNS-disease.
Since bulky disease is a common feature of T-LBL many patients ended up with a residual mass after treatment, most commonly, in the mediastinum. This clinical challenge has been approached with the addition of mediastinal irradiation in earlier studies. In the study by Thomas et al. [Citation13] patients treated with prophylactic mediastinal irradiation (30–39 Gy) after hyper-CVAD induction had lower incidence of mediastinal relapse compared to patients that received no irradiation. The benefits of mediastinal irradiation might however be related to specific induction treatments or irradiation dose since in a study by Hoelzer et al. [Citation10] no beneficial effect was seen. In that study, patients treated with GMALL-protocols received prophylactic mediastinal irradiation at a lower dose (24 Gy) but still the majority of relapses occurred in the mediastinum. Although none of the regimens precluded mediastinal irradiation only four patients received this as part of the primary treatment in our series. All four patients were treated with hyper-CVAD, and when comparing time to relapse/progression with non-irradiated patients treated with hyper-CVAD, there was a statistically significant difference in favor of the irradiation group. Although the number of patients is small, our results support the notion that there may be a beneficial effect of mediastinal irradiation at least for patients treated with hyper-CVAD chemotherapy.
The use of PET-CT as part of the evaluation and prediction for the risk of relapse is not very well described in T-LBL. In our cohort PET-CT was part of the induction response evaluation for 13 of the intensively treated patients, mostly because of residual masses. All the examinations were interpreted as normal, but still more than half of the patients relapsed. The PET-CT was not performed in a uniform manner, as exact time point for evaluation varied between patients and there was no central review or centralized protocol. These facts limits conclusions to be drawn from the results but underscores that PET-CT must be interpreted with caution and should be further investigated in clinical trials before it can be used for directing therapeutic decisions in T-LBL.
In conclusion our results show the beneficial effect of ALL-type treatment compared to CHOP-like therapy also in a completely unselected patient cohort. The results strongly suggests that all reasonably fit patients, including patients above 60 years of age, should be considered for intensive treatment as age had no impact on the risk for shorter survival. Also the addition of mediastinal radiation therapy should be considered for patients treated with hyper-CVAD. Relapse was the main reason for treatment failure and with the lack of targeted therapy, the role of even more intensified treatment for the youngest adult patients warrant further investigation.
Acknowledgments
The authors like to thank all colleagues contributing to the Swedish Lymphoma Registry and Lars Brudin for statistical advice.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brunning RD, Borowitz M, Matutes E, Head D, Flandrin G, Swerdlow SH, et al. Precursor T lymphoblastic leukaemia/lymphoblastic lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization. International Agency for Research on Cancer. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2001. p 115–7.
- Callens C, Baleydier F, Lengline E, Ben Abdelali R, Petit A, Villarese P, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol 2012; 30:1966–73.
- Raetz EA, Perkins SL, Bhojwani D, Smock K, Philip M, Carroll WL, et al. Gene expression profiling reveals intrinsic differences between T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Pediatr Blood Cancer 2006; 47:130–40.
- Sekimizu M, Sunami S, Nakazawa A, Hayashi Y, Okimoto Y, Saito AM, et al. Chromosome abnormalities in advanced stage T-cell lymphoblastic lymphoma of children and adolescents: A report from Japanese Paediatric Leukaemia/ Lymphoma Study Group (JPLSG) and review of the literature. Br J Haematol 2011;154:612–7.
- Nathwani BN, Diamond LW, Winberg CD, Kim H, Bearman RM, Glick JH, et al. Lymphoblastic lymphoma: A clinicopathologic study of 95 patients. Cancer 1981;48: 2347–57.
- Reiter A, Schrappe M, Ludwig WD, Tiemann M, Parwaresch R, Zimmermann M, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: A BFM group report. Blood 2000;95:416–21.
- Song KW, Barnett MJ, Gascoyne RD, Chhanabhai M, Forrest DL, Hogge DE, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol 2007;18:535–40.
- Sweetenham JW, Santini G, Qian W, Guelfi M, Schmitz N, Simnett S, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: Results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol 2001;19:2927–36.
- Levine JE, Harris RE, Loberiza FR, Jr., Armitage JO, Vose JM, Van Besien K, et al. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood 2003;101:2476–82.
- Hoelzer D, Gokbuget N, Digel W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002;99:4379–85.
- Dabaja BS, Ha CS, Thomas DA, Wilder RB, Gopal R, Cortes J, et al. The role of local radiation therapy for mediastinal disease in adults with T-cell lymphoblastic lymphoma. Cancer 2002;94:2738–44.
- Hunault M, Truchan-Graczyk M, Caillot D, Harousseau JL, Bologna S, Himberlin C, et al. Outcome of adult T-lymphoblastic lymphoma after acute lymphoblastic leukemia-type treatment: A GOELAMS trial. Haematologica 2007;92:1623–30.
- Thomas DA, O’Brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004;104:1624–30.
- Burkhardt B, Reiter A, Landmann E, Lang P, Lassay L, Dickerhoff R, et al. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: A report from the berlin-frankfurt-muenster group. J Clin Oncol 2009;27:3363–9.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86.
- Wollner N, Burchenal JH, Lieberman PH, Exelby P, D’Angio G, Murphy ML. Non-Hodgkin’s lymphoma in children. A comparative study of two modalities of therapy. Cancer 1976;37:123–34.
- Gustafsson G, Schmiegelow K, Forestier E, Clausen N, Glomstein A, Jonmundsson G, et al. Improving outcome through two decades in childhood ALL in the Nordic countries: The impact of high-dose methotrexate in the reduction of CNS irradiation. Nordic Society of Pediatric Haematology and Oncology (NOPHO). Leukemia 2000;14:2267–75.
- ClinicalTrials.gov [Internet]. Prednisolone or dexamethasone combined with chemotherapy in treating young patients with newly diagnosed lymphoblastic lymphoma. [cited 2013 April 15]. Available from: http://clinicaltrials.gov/
- Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006;107:1116–23.
- Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000;18:547–61.
- Hallbook H, Simonsson B, Ahlgren T, Bjorkholm M, Carneskog J, Grimfors G, et al. High-dose cytarabine in upfront therapy for adult patients with acute lymphoblastic leukaemia. Br J Haematol 2002;118:748–54.
- Chang MH, Kim SJ, Kim K, Oh SY, Lee DH, Huh J, et al. Clinical features and treatment outcomes of adult B- and T-lymphoblastic lymphoma: Results of multicentre analysis in Korea. Leuk Lymphoma 2009;50:1119–25.
- Lamvik J, Waage A, Wahl SG, Naess I, Paulsen PQ, Hammerstrom J. Adult acute lymphoblastic leukemia, Burkitt’s lymphoma and lymphoblastic lymphoma in middle Norway 1985–2004. Haematologica 2006;91:1428–9.
- Suzumiya J, Ohshima K, Kikuchi M, Takeshita M, Akamatsu M, Tashiro K. Terminal deoxynucleotidyl transferase staining of malignant lymphomas in paraffin sections: A useful method for the diagnosis of lymphoblastic lymphoma. J Pathol 1997;182:86–91.