Abstract
Trismus, a well-known sequelae after treatment of head and neck cancer, decreases a patient's oral function and quality of life. The main objectives of this study were to: 1) investigate the long-term prevalence of radiation-induced trismus in patients treated for head and neck cancer according to two different fractionation schedules; and 2) model a dose–response relationship for trismus.
Material and methods. Patients were recruited from the Swedish ARTSCAN trial, a prospective randomised multicentre study comparing conventional and accelerated fractionation. A total of 124 patients agreed to a clinical ENT examination 21–127 months (median 66 months) after beginning radiation therapy. Trismus-related scores were assessed using the EORTC H&N35 Quality of Life questionnaire. The TheraBite® range of motion scale was used to measure maximal interincisal distance. The dose–response relationship for structures important for mastication and the temporomandibular joints was investigated by normal tissue complication probability modelling.
Results. No significant differences in patient-reported trismus or maximal interincisal distance were found between the two trial arms. Patient-reported moderate to high scores regarding trismus increased from 3% at the start of radiation therapy to 25% at the long-term follow-up. Maximal interincisal distance correlated significantly with patient-reported scores of trismus. The best dose–response fit to the endpoint data was found for the dose to the ipsilateral masseter.
Conclusions. Trismus is a persistent complication after radiotherapy with 3D-conformal radiation therapy. We found no difference between the severity and prevalence of trismus between conventional and accelerated fractionation, but a significant correlation between the absorbed dose to the mastication structures and opening of the mouth. Further prospective studies may determine whether a reduced dose to structures important for mastication using intensity-modulated radiation therapy will reduce problems with trismus.
Trismus, defined as reduced mandible mobility, is a well-known complication of radiation therapy (RT) in the treatment of head and neck cancer. The reported prevalence of trismus varies considerably, from 5% for intensity-modulated radiation therapy (IMRT) [Citation1,Citation2] to between 25% and 45% for three-dimensional conformal radiation therapy (3D-CRT) [Citation1,Citation3,Citation4]. This large range is due not only to treatment technique but to lack of uniform criteria for defining trismus, varying assessment methods, surgical procedures, adjuvant chemotherapy, different tumour sites, and study designs [Citation5]. Trismus has a negative impact on quality of life (QoL) [Citation3], as it makes chewing, eating, speaking, and personal dental hygiene more difficult and obstructs the delivery of proper dental care [Citation3], increasing the risk of osteoradionecrosis. Trismus may also render the assessment of local tumour control more difficult. It is frequently overlooked during treatment as many patients receiving RT, chemotherapy, or both, require feeding tubes or choose to restrict their intake to liquids during and after treatment.
The ARTSCAN study is a multicentre study of 750 randomised patients receiving conventional versus accelerated RT for head and neck squamous cell carcinoma. Details of the trial's design and the results two years after the last patients were included in ARTSCAN were recently published [Citation6]. The principal aims of our investigation were to evaluate the prevalence and severity of radiation- induced trismus in a cohort of patients in the ARTSCAN study by means of patient-reported QoL and measurements of maximum mouth opening; and assess whether there was a significant difference between the two treatment schedules. An additional objective was to evaluate the dose– response relationship of structures important for the development of trismus by employing normal tissue complication probability (NTCP) modelling.
Material and methods
Patients and treatment delivery
The patients in our study were recruited from the Swedish ARTSCAN trial, a prospective randomised multicentre trial comparing radical RT (68 Gy) with conventional fractionation (CF) administered over the course of seven weeks; and accelerated fractionation (AF) delivered with a concomitant boost technique at the same total dose over 4.5 weeks for squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx [Citation6,Citation7]. No chemotherapy was administered three months prior to or during RT. CT-based 3D treatment planning was mandatory. Gross tumour volume and both clinically involved and uninvolved lymph nodes (PTV-A) received 46 Gy, while gross tumour volume and clinically involved lymph nodes (PTV-B) received an additional 22 Gy. AF was delivered as a boost with 1.10 Gy/fraction in 20 fractions at the same time PTV-A was being treated. The inter- fraction interval in the AF arm was never shorter than six hours. Further details on the treatment technique and quality assurance in the trial may be found in Johansson et al. [Citation7].
The patients in the present sub-sample were recruited from two regions, the north and the south of Sweden, where 380 of the 750 ARTSCAN patients had been randomised. Fifteen months after ARTSCAN was concluded, we invited patients who participated in the study to an extensive clinical ENT examination. At this time 207 of the 380 patients in our original cohort were alive. Of these, 124 who were free of recurrent local disease agreed to attend. Baseline characteristics at the time of inclusion in the ARTSCAN trial for those in the present study are shown in . One hundred and fifteen patients (93%) had been treated with 3D-CRT technique, while IMRT was used on the remaining nine individuals. Fifteen patients underwent post-RT surgery of the primary tumour, 47 neck dissection, and eight combined surgery of the primary tumour region and neck dissection.
Table I. Baseline characteristics at time of randomisation in the ARTSCAN study, conventional fractionation (CF) versus accelerated fractionation (AF).
This study was approved by the regional ethical review board in Umeå, Sweden (EPN Umeå Dnr 07-023M). Informed consent forms were signed by all participants.
ENT examination and assessment of maximal interincisal distance of mouth opening
The ENT examination was performed at a median of 66 months (range 21–127) after the initiation of RT. The examination included measuring the greatest extent of mouth opening as maximal interincisal distance (MID) in millimetres with the TheraBite® Range of Motion Scale (Atos Medical AB). Data was collected for 121 patients (missing n = 3; all treated with 3D-CRT).
Patients were asked to open their mouths as wide as possible and the MID of the frontal teeth was measured. Four edentulous patients did not use any dentures, and three only wore a single denture in the upper jaw. Another three patients who were edentulous in the lower jaw also did not use a denture. The MID in these patients was measured as the distance from the alveolar ridge to the incisal edge of the incisor of the jaw, or to the opposing alveolar ridge, as described by Dijkstra et al. [Citation8]. No accepted criterion for trismus appears in the literature. We used MID ≤ 35 mm as the limit for trismus, as proposed by the aforementioned authors.
Quality of life
QoL was measured at the ENT examination using the EORTC H&N35 Quality of Life questionnaire, which had also been used in the ARTSCAN study [Citation9]. Data from the ARTSCAN database was employed for longitudinal studies of trismus-related QoL questions. In the ARTSCAN study QoL was measured several times: before treatment began, after RT was completed, and at 3, 6, 12, 24, and 60 months after the start of treatment. Two of the EORTC QLQ-H&N35 questions, “Have you had pain in your jaw?” (Q32), and “Have you had problems opening your mouth wide?” (Q40), were particularly studied. Both had four answer alternatives: 1) “Not at all”, 2) “A little”, 3) “Quite a bit”, and 4) “Very much”.
NTCP modelling
DICOM-RT data were retrieved from the ARTSCAN DICOM database (121 treatment plans were available for the present patient cohort). All treatment plans had been produced with the Helax-TMS Version 6.1 treatment planning system (TPS) (Nucletron, The Netherlands). Dose distributions were calculated by means of its pencil beam algorithm with correction for inhomogeneities activated. The majority of the patients who had complete DICOM data were treated with 3D-CRT (112/121), while the remaining patients were treated with IMRT or a combination of 3D-CRT and IMRT. Slice thickness of the treatment planning CT was ≤ 5 mm for 103 patients, and 10 mm for 18 patients treated early in the study.
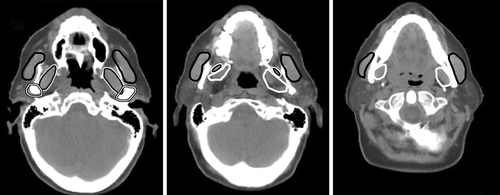
The mastication structures chosen for the NTCP modelling were the masseter, the medial and lateral pterygoid muscles, and the temporomandibular joints (TMJ). They were identified and delineated on each axial CT slice () in the TPS. Muscle tissue was delineated without a margin. The segmentation of the TMJ included the capsule around the bony structure, the condylar process and the mandibular fossa. The same physician (EK) did the contouring for all patients.
Figure 1. Delineated structures important for mastication in three different CT slices: masseter (black line), lateral (black line with white outline), and medial (white line) pterygoid muscles, and temporomandibular joints (white line with black outlines).
The CT images, new structure sets, and original DICOM dose matrices from Helax-TMS were then imported into the CERR software package [Citation10] for final retrieval of dose–volume data. Doses were computed separately for the ipsilateral and contralateral structures, and for the paired structures. In addition, the analysis was performed for the mastication muscles combined into a single structure on the ipsilateral and contralateral side, respectively.
A logistic function with the physical mean dose (i.e. uncorrected for fractionation effects) for each structure was used for the NTCP calculations:
where D50 is the dose value at which the probability of complication is 50%, and γ is the normalised dose gradient at D50. NTCP was calculated for two endpoints: Q40 (“problems opening mouth wide”) and MID. Endpoint data were dichotomised with QoL scores 3–4 and MID ≤ 35 mm defining the presence of the complication.
Statistical methods
Student's t-test for independent samples was used to analyse differences between groups with continuous variables, while the Pearson's χ2-test was applied to categorical data. The analysis of the correlation between mean doses and outcome data was performed with Spearman's rank correlation coefficient. All tests were two-sided and p-values less than 0.05 were considered significant.
The NTCP model was fitted to patient data using maximum likelihood analysis; 95% confidence intervals for D50 and γ50 were calculated using profile likelihood estimation. Receiver operating characteristics (ROC) analysis was used to test the predictive power of the model. Cross validation using leave-one-out (CV-LOO) analysis was performed to test the robustness of the parameter predictions.
Results
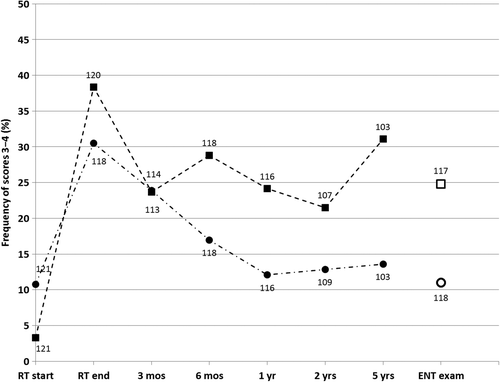
Before beginning RT, 4/121 patients (3%) reported having “quite a bit” to “very much”, i.e. scores 3 or 4 on Q40 (“problems opening mouth wide”). Moreover, 13 patients (11%) reported scores of 3 or 4 on Q32 (“jaw pain”). The corresponding numbers immediately after completing RT were 46/120 (38%) for Q40 and 36/118 (31%) for Q32. At the long-term follow-up the corresponding figures were 29/117 (25%) for Q40 and 13/118 (11%) for Q32. The longitudinal results from the ARTSCAN database for these QoL questions and the ENT examination at the long-term follow-up are presented in . There was a significant improvement in “problems opening mouth wide” at three months compared to the termination of RT (p = 0.04); it then levelled off at 25% with no further change of any significance. The incidence of “jaw pain” decreased significantly (p = 0.002) from the completion of RT and until one year thereafter, when it reached its baseline value.
Figure 2. Frequency of scores 3 − 4 for Quality of Life questions (QoL) Q40 “problems opening mouth wide” (squares) and Q32 “jaw pain” (circles). Longitudinal data from ARTSCAN database (filled markers) and from ENT examination (open markers) at median 66 months after start of radiation therapy. Labels at markers indicate number of answers to QoL questionnaire at each follow-up and at ENT examination.
The results of the MID measurements and trismus (as defined earlier) in the sub-sample of patients from the two treatment arms are presented in . In total, 50/121 (41%) had trismus (MID ≤ 35 mm) at the time of the follow-up ENT examination. There was no significant difference between the two treatment schedules. This was true for all MID cut-off values in the range 20–40 mm.
Table II. Maximal interincisal distance measures (MID) and trismus for conventional fractionation (CF) versus accelerated fractionation (AF).
We found a strong inverse correlation (r = -0.67, p < 0.0001) between MID and patient-reported scores for Q40 “problems opening mouth wide” and a weak inverse correlation for Q32 “jaw pain” (r = −0.24, p = 0.009). The mean MID for scores 1–2 on Q40 was 41.4 mm, and 27.8 mm for scores 3–4 (p < 0.0001). Corresponding results for scores 1–2 on Q32 were 38.7 mm, and 33.1 mm for scores 3–4, showing no significant difference (p = 0.08). Patients with oral and oropharyngeal tumours had a significantly lower average MID (35.7 mm) compared to patients with laryngeal and hypopharyngeal tumours (46.7 mm) (p < 0.0001). No correlation was found between MID and patient age, sex, tumour stage, nodal status, clinical stage, post-surgery of primary tumour or neck dissection, or performance according to Karnofsky index.
An inverse correlation between MID and the mean dose to all the delineated mastication structures was found with the strongest correlation indicated for the ipsilateral structures, i.e. those receiving the highest mean dose. The single structure with the strongest correlation was the ipsilateral masseter muscle (r = -0.49, p < 0.0001). The correlation decreased for the ipsilateral pterygoid muscles (r = -0.43) and the TMJ (r = -0.38). The correlation between MID and mean dose was weaker if the “paired structures” were considered (average r = -0.37) and weaker still for the corresponding contralateral structures (average r = −0.25).
The NTCP modelling showed the same pattern. The best fit was for the mean dose to the ipsilateral masseter for both Q40 and MID 35 mm (), with D50/γ equal to 72.3 Gy/1.04 for Q40 and 57.2 Gy/0.78, for MID 35 mm. The resulting NTCP curves are presented in for both endpoints. When combining the mastication muscles into a single structure (one for the ipsilateral and one for the contralateral side), the dose–response parameters D50 and γ, as well as the area under the ROC curve, Az, were similar to the data for the masseter (see ).
Figure 3. Normal tissue complication probability (NTCP) versus mean dose to ipsilateral masseter for endpoints (a) Q40 “problems opening mouth wide” (scores 3–4) and (b) maximal interincisal distance MID ≤ 35 mm. Line represents modelled logistic function and squares are measured frequencies for 15 Gy dose bins. Circles indicate distribution of non-responders (NTCP = 0) and responders (NTCP = 1).
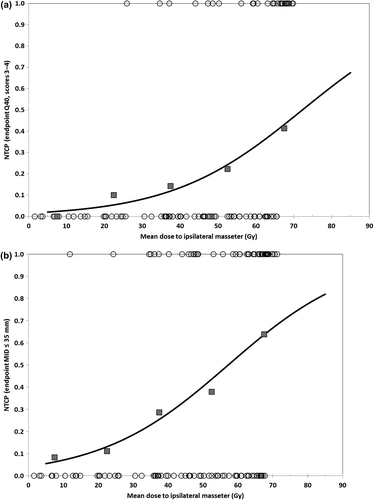
Table III. Normal tissue complication probability (NTCP) modelling data for endpoints Q40 “problems opening mouth wide” (n = 114) and maximal interincisal distance MID ≤ 35 mm (n = 118) for ipsilateral mastication muscles and temporomandibular joint (TMJ). Maximum log-likelihood values (LLmax) and parameter estimates with 95% profile likelihood confidence intervals (CIs) and area under receiver operating characteristics (ROC) curve (Az) with standard error of the mean (SEM) for parameter estimates at LLmax.
The CV-LOO analysis showed that the NTCP parameter predictions and area under the ROC curve values were robust. The mean values for D50, γ50 and Az for all tested cohorts were equal to the results for the whole patient group (within the precision given in ) with relative standard deviations of less than 1.5%, 3.5% and 0.7%, respectively, for all structures. The ranking of the Az values for the ipsilateral mastication muscles was the same as presented in , i.e. masseter, mastication structures combined, lateral and medial pterygoid in descending order, for every tested cohort. The differences in Az values for the ipsilateral medial pterygoid muscle and the ipsilateral TMJ were small in all test cohorts (mean values not significantly different) which was also the case in the original data set.
Discussion
The purpose of the present study was to evaluate the late effects of CF and AF on structures important for the development of trismus in a sub-sample of patients in the ARTSCAN study. Very few patients experienced problems with trismus before they began RT, although most of them had an oral or oropharyngeal cancer (78%). We found a strong correlation (p < 0.0001) between MID and patient-reported scores on problems with opening the mouth. The percentage of patients reporting such difficulties who also had MID ≤ 35 mm at the long-term follow-up corresponds with other studies using 3D-CRT [Citation1,Citation3]. No difference in prevalence or severity of trismus in the two treatment arms could be detected. Trismus is a late normal tissue complication. The difference in the equivalent dose at 2 Gy (EQD2) between the CF arm at 68 Gy and the AF arm at 64 Gy during the trial (assuming α/β = 3 Gy and complete repair between fractions) is likely to be too small to result in any detectable difference in outcome at a long-term follow-up.
Trismus is probably caused by radiation-induced damage to the neuromuscular structures important for mastication, and to a lesser extent damage to the TMJ, resulting in radiation-induced fibrosis. The mechanisms behind the development of this fibrosis are complex and not completely understood, but they may result in permanent muscular spasm, and sclerosis of the muscles involved in mastication [Citation11,Citation12].
There was a strong correlation between the mean doses of the separate mastication structures. This makes it difficult to draw firm conclusions regarding each structure's influence on the development of post-radiation trismus. The best NTCP fit for a single structure in our patient population was found for the ipsilateral masseter. An equally good fit was obtained if all ipsilateral muscles were considered as a single organ. The NTCP calculations also provided a better fit between predicted and observed MID/Q40 for the dose to the mastication muscles than to the TMJ ().
Teguh et al. reported that for every 10 Gy increase in dose above 40 Gy to the pterygoid muscle, the probability of trismus increases by 24% [Citation13]. Several other studies have also found a stronger correlation between post-radiation trismus and the absorbed dose to the mastication muscles rather than to the TMJ [Citation12,Citation14–16]. This is in accordance with our results.
We also examined if the prediction was improved in a multivariate analysis, using logistic regression. Once the mean dose for the ipsilateral masseter was entered into the equation, none of the other calculated structure doses or clinical variables () contributed significantly (p > 0.05) to the model.
One major consideration is the definition of trismus. Earlier definitions describe it as a muscular spasm that restricts the opening of the mouth. We have defined trismus as a reduced ability to open the mouth fully regardless of the cause, with the cut-off point at MID ≤ 35 mm as suggested by Dijkstra et al. [Citation8]. The Lent Soma tables [Citation17] define trismus as MID at 20 mm and less. If we had used MID ≤ 20 mm as a cut-off value, only 8% of our patients would have fit the criteria for trismus. In the CTCAE 4.02 Adverse Event, Trismus Grade 1 is defined as “‘Decreased ROM (range of motion) without impaired eating” and Grade 2 as “Decreased ROM requiring small bites, soft foods or purees”, but a cut-off value for MID is not specified [Citation18]. In our material we found no significant difference in incidence of trismus between AF and CF for any cut-off value in the range 20–40 mm.
Our longitudinal patient-reported data show that the incidence of trismus has a peak around the termination of RT. It then decreases and stabilises about three months after RT. Different treatment modalities have been suggested. Physiotherapy using various external opening devices is generally considered the main treatment for trismus, but evidence of its effectiveness is not convincing [Citation11,Citation19,Citation20]. Pentoxyphilline was reported to have some positive effects on trismus, as did coronoidectomy in head and neck cancer patients with severe trismus [Citation21,Citation22]. It is reasonable to argue for a focus on prevention of trismus during treatment, with optimised RT planning, as also concluded in a recent review [Citation23].
With modern IMRT technique it is often possible to reduce the absorbed dose to structures important for mastication, depending on the site and volume of the tumour. In the present cohort, only nine patients received IMRT for PTV-A (prophylactic volume), and five of them also received it for PTV-B (gross tumour volume with 2 cm margin). However, a reduced prevalence of trismus has been reported with modern IMRT technique [Citation2,Citation24,Citation25].
In this work we have presented NTCP data based on the mean dose to the relevant structures rather than, e.g. VD (the relative volume V of a structure receiving a dose D or higher). There is a very strong correlation between the mean dose and VD in our patient material, especially in the range V40Gy–V60Gy, with correlation coefficients close to 0.95 for the investigated structures. If we apply VD within this range rather than the mean dose in the NTCP modelling the predicted power of the model, measured as the area under the ROC curve, is very similar to what we obtain for the mean dose. However, the rather large CT slice thickness used in our study limits the accuracy of structure delineation, especially for small structures like the TMJ. This, in combination with a rather coarse TPS dose calculation grid (5 mm for most of the cases) probably makes the mean dose a more robust dose measure for our patients than more sophisticated dose–volume descriptors. Hence we have only reported NTCP-calculations using mean doses. Van der Molen et al. [Citation16] also found several investigated dose–volume descriptors (mean dose, max dose, V20Gy, V40Gy and V60Gy) of the masseter and pterygoid muscles to be significant predictors of trismus (subjective restricted mouth-opening) at one year after chemo-IMRT. They conclude that it is essential to reduce the dose to these structures when planning the treatment, especially the masseter muscle, which is in accordance with our findings.
Eighty-three (40%) of the 207 potential patients fulfilling the inclusion criteria abstained from taking part in the study, which might introduce bias in the generalisation of the results. However, the baseline characteristics at the time of randomisation in the ARTSCAN study (age, gender, Karnofsky index, tumour site, T-stage or nodal status) were not significantly different between the 124 participating patients compared to the 83 non-participating patients. More important, no significant differences between the two groups were found for questions, Q40 (“problems opening mouth wide”) and Q32 (“jaw pain”) for scores 1–2 versus 3–4, neither before start of RT nor at two or five years after RT.
The lack of MID baseline data is a drawback in our study. The baseline data for QoL question Q40 shows however that the number of pre-RT reported scores 3–4 (moderate to severe) for this QoL question is low, only 3% (see ). We have also shown a strong inverse correlation between patient-reported scores and MID. It is therefore reasonable to assume that the prevalence of trismus was low before start of RT. No difference in trismus incidence in the two trial arms and non-significant changes in the NTCP-modelling parameters were found when we repeated the analysis including only patients reporting no problems at all (score 1) pre-RT for Q40, which strengthens this assumption.
In conclusion our study found no significant difference between the prevalence and severity of radiation-induced trismus after definitive RT with CF and AF. There was a high prevalence of long-term trismus after 3D-CRT, as indicated by assessing the MID of the mouth opening. Patient-reported scores on ability to open the mouth correlated significantly with clinical findings. We also found a significant correlation between trismus and the absorbed dose to mastication structures, especially the ipsilateral masseter muscle. It would be valuable to determine whether using IMRT at a reduced dose on structures important for mastication decreases trismus.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by grants from the Swedish Cancer Society, the Acta Oto-Laryngologica Foundation, and Finnmark Health Trust.
References
- Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer 2010;18:1033–8.
- Chen YY, Zhao C, Wang J, Ma HL, Lai SZ, Liu Y, et al. Intensity-modulated radiation therapy reduces radiation-induced trismus in patients with nasopharyngeal carcinoma: A prospective study with > 5 years of follow- up. Cancer 2011;117:2910–6.
- Pauli N, Johnson J, Finizia C, Andréll P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol 2013;52:1137–45.
- Louise Kent M, Brennan MT, Noll JL, Burri SH, Hunter JC, Lockhart PB. Radiation-induced trismus in head and neck cancer patients. Support Care Cancer 2008; 16:305–9.
- Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: A systematic review. Oral Oncol 2004;40:879–89.
- Zackrisson B, Nilsson P, Kjellén E, Johansson KA, Modig H, Brun E, et al. Two-year results from a Swedish study on conventional versus accelerated radiotherapy in head and neck squamous cell carcinoma—the ARTSCAN study. Radiother Oncol 2011;100:41–8.
- Johansson KA, Nilsson P, Zackrisson B, Ohlson B, Kjellén E, Mercke C, et al. The quality assurance process for the ARTSCAN head and neck study – a practical interactive approach for QA in 3DCRT and IMRT. Radiother Oncol 2008;87:290–9.
- Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg 2006;35:337–42.
- Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H & N35. J Clin Oncol 1999;17:1008–19.
- Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys 2003; 30:979–85.
- Stubblefield MD, Manfield L, Riedel ER. A preliminary report on the efficacy of a dynamic jaw opening device (dynasplint trismus system) as part of the multimodal treatment of trismus in patients with head and neck cancer. Arch Phys Med Rehabil 2010;91:1278–82.
- Bhatia KS, King AD, Paunipagar BK, Abrigo J, Vlantis AC, Leung SF, et al. MRI findings in patients with severe trismus following radiotherapy for nasopharyngeal carcinoma. Eur Radiol 2009;19:2586–93.
- Teguh DN, Levendag PC, Voet P, van der Est H, Noever I, de Kruijf W, et al. Trismus in patients with oropharyngeal cancer: Relationship with dose in structures of mastication apparatus. Head Neck 2008;30:622–30.
- Krasin MJ, Wiese KM, Spunt SL, Hua CH, Daw N, Navid F, et al. Jaw dysfunction related to pterygoid and masseter muscle dosimetry after radiation therapy in children and young adults with head-and-neck sarcomas. Int J Radiat Oncol Biol Phys 2012;82:355–60.
- Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: A prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:365–73.
- van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CR, et al. Dysphagia and trismus after concomitant chemo-intensity-modulated radiation therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol 2013;106:364–9.
- LENT SOMA tables. Radiother Oncol 1995;35:17–60.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.02. 2009-05-28 ed. City of Publication: National Institutes of Health; 2009.
- Dijkstra PU, Sterken MW, Pater R, Spijkervet FK, Roodenburg JL. Exercise therapy for trismus in head and neck cancer. Oral Oncol 2007;43:389–94.
- Ahlberg A, Engstrom T, Nikolaidis P, Gunnarsson K, Johansson H, Sharp L, et al. Early self-care rehabilitation of head and neck cancer patients. Acta Otolaryngol 2011; 131:552–61.
- Chua DT, Lo C, Yuen J, Foo YC. A pilot study of pentoxifylline in the treatment of radiation-induced trismus. Am J Clin Oncol 2001;24:366–9.
- Bhrany AD, Izzard M, Wood AJ, Futran ND. Coronoidectomy for the treatment of trismus in head and neck cancer patients. Laryngoscope 2007;117:1952–6.
- Wranicz P, Herlofson BB, Evensen JF, Kongsgaard UE. Prevention and treatment of trismus in head and neck cancer: A case report and a systematic review of the literature. Scand J Pain 2010;1:84–8.
- Hsiung CY, Huang EY, Ting HM, Huang HY. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: The reduction of radiation-induced trismus. Br J Radiol 2008;81:809–14.
- Chao KS, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: Impact of tumor volume. Int J Radiat Oncol Biol Phys 2004;59:43–50.
