Abstract
Purpose. The aim of this work was to investigate cardiorespiratory fitness in breast cancer patients at different time points of anti-cancer treatment.
Patients and methods. Non-metastatic breast cancer patients (n = 222, mean age 55 years) were categorized into four subgroups according to their treatment status. Cardiopulmonary exercise testing (CPET) was used to measure patients’ cardiorespiratory fitness, including oxygen delivery and metabolic muscle function. Testing was performed by bicycle ergometry, and maximal oxygen uptake (VO2peak) was measured. Heart rate during exercise at 50 watts (HR50) was assessed as a cardiocirculatory parameter and ventilatory threshold (VT) was used as an indicator of the O2 supply to muscle. Analysis of covariance was used to estimate the impact of different cancer treatments on cardiorespiratory fitness with adjustment for clinical factors.
Results. Submaximal measures were successfully assessed in 220 (99%) and 200 (90%) patients for HR50 and VT, while criteria for maximal exercise testing were met by 176 patients (79%), respectively. The mean VO2peak was 20.6 ± 6.7 ml/kg/min, mean VT 10.7 ± 2.9 ml/min/kg and mean HR50 112 ± 16 beats/min. Chemotherapy was significantly associated with decreased VO2peak, with significantly lower adjusted mean VO2peak among patients post adjuvant chemotherapy compared to patients with no chemotherapy or those who just started chemotherapy regime (all p < 0.01). Patients post adjuvant chemotherapy reached only 63% of the VO2peak level expected for their age- and BMI-category (mean VO2peak 15.5 ± 4.8 ml/kg/min). Similarly, HR50 was significantly associated with treatment. However, VT was not associated with treatment.
Conclusion. Breast cancer patients have marked and significantly impaired cardiopulmonary function during and after chemotherapy. Hereby, chemotherapy appears to impair cardiorespiratory fitness by influencing the oxygen delivery system rather than impacting metabolic muscle function. Our findings underline the need of exercise training in breast cancer patients to counteract the loss of cardiorespiratory fitness during the anti-cancer treatment.
Breast cancer-specific survival has substantially improved over the past decades. In the US, the relative survival rate is about 89% at five years after breast cancer diagnosis [Citation1]. Nevertheless, there are concerns about morbidity and mortality, particularly related to cardiovascular fitness. To date, cardiovascular disease mortality is more common among breast cancer survivors than breast cancer-related mortality among women who were 66 years or older [Citation2]. An important predictor of cardiovascular events and all-cause mortality is cardiorespiratory fitness, as shown in healthy individuals and clinical populations including breast cancer patients after anti-cancer treatment [Citation3,Citation4]. Poor cardiorespiratory fitness also has direct consequences on the performance of everyday tasks and thus impacts quality of life.
The gold standard of measuring cardiorespiratory fitness is cardiopulmonary exercise testing (CPET) [Citation5]. This method determines the overall cardiovascular and respiratory function during exercise, yielding the peak oxygen uptake (VO2peak) as a maximal performance measure. In cancer patients, the oxygen system can be adversely affected by chemotherapy. Effects of chemotherapeutic agents on the respiratory, cardiac, blood, vascular, or skeletal muscle functions have been observed or hypothesized, potentially contributing to the impairment of cardiorespiratory fitness [Citation6]. Cardiorespiratory function is not routinely measured at any stage of breast cancer treatment, and CPET is scarcely used in clinical settings [Citation7]. Although low VO2peak measures have been observed in intervention studies across the breast cancer survivorship continuum [Citation8], to our knowledge, there is very limited information on the impact of cancer therapy on cardiorespiratory fitness using gold standard testing methods.
Therefore, this study investigated maximal and submaximal cardiorespiratory fitness parameters among 222 non-metastatic breast cancer patients at the beginning of chemotherapy, after completion of chemotherapy, or without chemotherapy, using the gold standard for cardiorespiratory measurement. We compared cardiorespiratory capacity of these patient groups adjusted for clinical relevant parameters and compared them to healthy women with the same age and BMI. The role of cancer therapy was investigated.
Patients and methods
Setting and participants
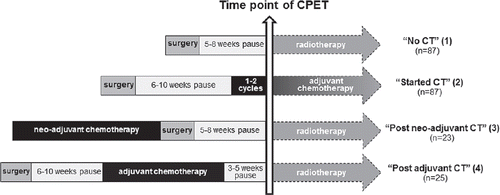
The data for this analysis were data at enrollment into two randomized controlled exercise trials (baseline testing), the BEATE-Study and the BEST-Study [Citation9,Citation10]. The studies investigated the effects of a 12-week progressive resistance versus relaxation training in breast cancer patients undergoing chemotherapy (BEATE-Study) or radiotherapy (BEST-Study). Besides the different treatment modalities, the inclusion/exclusion criteria were equal in both studies. Exact inclusion and exclusion criteria and more details of the trials are presented elsewhere [Citation9,Citation10]. Included in this analysis were all participants who had completed the baseline assessment by November 2012 (n = 222). Both trials were conducted with parallel designs at the National Center for Tumor Diseases (NCT) in Heidelberg, Germany. The University of Heidelberg Ethics Committee has approved both trials and written informed consent was obtained for all procedures. Women with histologically confirmed stage 0-III primary breast cancer after lumpectomy or mastectomy were eligible for the study. Participants recruited in the BEST-Study had the baseline CPET within 14 days before start of radiotherapy. Of these participants, a majority had not received chemotherapy (n = 87), while some had received chemotherapy in the adjuvant (n = 25) or neo-adjuvant (n = 23) chemotherapy setting. Patients enrolled in the BEATE-Study (n = 87) performed baseline CPET at the end of the first or second chemotherapy cycle. Based on the different treatment histories, four subgroups (see ) could be identified and were analyzed: 1) no chemotherapy; 2) started adjuvant chemotherapy; 3) post neo-adjuvant chemotherapy; 4) post adjuvant chemotherapy.
Cardiorespiratory fitness
CPET was performed using an electronically braked cycle ergometer (ergoselect 100, ergoline, Bitz, Germany). A stepwise incremental exercise protocol was applied starting at 50 watts with increments of 25 watts every two minutes until volitional exhaustion or medical reasons for exercise termination (see below) were reached. Gas exchange was measured using a breath-by-breath gas analysis system (ergostik, Geratherm Respiratory, Bad Kissingen, Germany) which was calibrated according to the instructions of the manufacturer before each test. To monitor patient safety, a 12-lead electrocardiogram (ECG) was applied (CardioPart 12 Blue, amedtec, Aue, Germany) and blood pressure was measured with a standard cuff sphygmomanometer at rest, in the middle of each exercise stage, and three times during the five minute recovery period. Exercise was terminated prematurely in the case of major ECG abnormalities, severe dyspnea or excessive RR increase (> 230 mmHg systolic and/or > 110 mmHg diastolic).
VO2peak values were included in the analyses that satisfied at least one of the following criteria for maximal effort was fulfilled: respiratory exchange ratio (RER) > 1.1 or peak heart rate (HRpeak) ± 10% beats of the age-appropriate reference value [Citation11,Citation12]. VO2peak values were considered in relation to body weight (ml/min/kg). In addition, absolute values (ml/min) were also given. Peak work rate, defined by the maximal power output (in watt) reached at the end of the highest exercise level, was interpolated if the last exercise stage was not maintained for two minutes. VO2peak and peak respiratory exchange ratio (RERpeak) were assessed as the highest 30 second average during exercise.
Submaximal exercise measures during CPET included the ventilatory threshold (VT) and heart rate at 50 watts (HR50). The VT was determined using the V-slope method according to Beaver et al. [Citation13] as primary criterion and the first rise in the ventilatory equivalent for oxygen (VE/VO2) as a secondary criterion [Citation14]. VT was rated independently by two experienced investigators; in case of different results, the raters re-evaluated the threshold together. The VT was also independently rated as “easily determinable”, “difficult to determine” or “indeterminable”. In the latter case, VT was not included in the analysis. In the analyses, VT values were considered relative to body weight (ml/min/kg). Resting HR and HR50 were obtained from the ECG recordings as five second averages in a sitting position before the start of exercise and at the end of the 50 watt stage, respectively.
Medical and patient demographics
Medical characteristics and treatment modalities were abstracted from medical records. Overall performance status was determined by the attending oncologist using the Eastern Cooperative Oncology Group (ECOG) performance score system at the time of enrollment. Weight and height were measured at baseline. Exercise behavior in the year before breast cancer diagnosis was assessed through self-developed surveys abstracted from the International Physical Activity Questionnaire. Participants were asked about the type, frequency, and duration of exercise (e.g. walking, cycling, and intentional exercise). Furthermore, exercise behavior during adolescence was also recorded classified as “competitive exercise” (for at least 3 years), “non-competitive exercise” or “none”.
Statistical analysis
Clinical and patient demographics, as well as fitness parameters were investigated by descriptive analyses for the entire study population, and also stratified by the four treatment subgroups. Between-group differences were assessed using χ2 or Fisher's exact test for categorical variables, and using one-way ANOVA for continuous variables. Expected VO2peak values were calculated for each woman from the regression formula presented by Koch et al. [Citation15], according to her BMI (< 25, ≥ 25 kg/m2) and age (in 5-year categories). Differences between the measured and the expected VO2peak values were investigated using paired t-tests. For each patient, the proportion of the observed versus expected VO2peak values was calculated.
Analyses of covariance (ANCOVA) models were used to test different fitness outcomes (i.e. VO2peak, VT, and HR50) with the categorical treatment variable (no chemotherapy/started chemotherapy/post neo-adjuvant chemotherapy/post adjuvant chemotherapy) as independent exposure of interest. The assumptions of homoscedasticity, normality of the residuals, and homogeneity of regression slopes were not violated. The models were adjusted for covariates selected on the basis of the theory of directed acyclic graphs [Citation16], i.e. age at diagnosis, pre-diagnosis BMI (17–< 25, 25 to < 30, ≥ 30 kg/m2), smoking status at diagnosis (current, former, never), sports in the year before diagnosis (none, > 0–15 MET*h/wk, > 15–35 MET*h/wk, > 35 MET*h/wk), sports during adolescence (none, non-competitive, competitive), walking (0–1 5 h/wk, > 1–3 5 h/wk, > 3–5 5 h/wk, > 5 h/wk) and cycling (none, > 0–1 h/wk, > 1–3 h/wk, > 3 h/wk) in the year before diagnosis, use of beta-blockers, or pre-existing cardiac diseases. Sensitivity analyses were performed by including hemoglobin level, trastuzumab treatment, hormone treatment, or type of chemotherapy (taxanes, anthracyclines), where the causal direction of the association with cardiopulmonary fitness is unclear. We also checked the parsimonious models including only significant covariates and those that changed the treatment estimate by > 10%, but there were no substantial changes in the results. In addition, type of chemotherapeutic agent was further evaluated among patients who have started chemotherapy using a model that included categorized the variable as taxane use only, anthracycline use only, or use of both.
Results
A total of 222 breast cancer patients with a mean age of 54.8 ± 9.3 years were included in the analyses. Characteristics of the population are presented in . Patients who received no chemotherapy were slightly older than those in the other treatment groups. Mean hemoglobin (Hb) values were within the normal range, of above 12 g/dl, with the exception of the group post adjuvant chemotherapy, where the mean Hb was 11.4 g/dl indicating NCI-grade 1 anemia. Overall, only two patients had a Hb value slightly below 10 g/dl, which is defined as NCI-grade 2 anemia.
Table I. Characteristics of the study population.
The mean duration of exercising in the CPET was 6 minutes 53 seconds, which is valid to determine the VO2peak [Citation17]. Of the sample population, 176 patients (79.3%) exercised until exhaustion and fulfilled the criteria for maximal effort, therefore were included in the analysis of maximal exercise measures. However, 46 patients (20.7%) did not fulfill the criteria for maximal effort. In 18 cases (8.1%), exercise was terminated prematurely for medical reasons: seven patients (3.2%) demonstrated an excessive increase in blood pressure, three patients (1.4%) demonstrated new ECG abnormalities, one (0.5%) patient experienced severe dyspnea, and seven patients (3.2%) experienced orthopedic problems, such as knee or back pain. However, no major adverse event occurred. An additional 28 patients (12.6%) reported problems with the face mask, had poor compliance or experienced other volitional reasons to terminate the CPET before exhaustion was reached. VT was indeterminable in 22 of 222 cases (9.9%) as a result of high/low ventilation or due to above mentioned medical abort criteria. In 109 cases (49.1%), VT was easily determinable, while 91 (41.0%) were deemed difficult to determine.
The peak and submaximal exercise measures are summarized in . The mean VO2peak was lowest among patients post adjuvant chemotherapy (15.5 ± 4.8 ml/min/kg) and highest among patients who had just started chemotherapy (23 ± 7.1 ml/min/kg). The VT was also highest among patients who had just started chemotherapy (11.4 ± 3.4 ml/min/kg), but did not differ substantially between the other three groups (p = 0.21). The mean HR50 was highest among those post adjuvant chemotherapy with a tendency of tachycardia in this group (see ) and lowest in the group without chemotherapy. The biggest increases from resting HR to HR50 were observed among patients in post neo-adjuvant and adjuvant chemotherapy groups.
Table II. Maximal (peak) and submaximal fitness parameters, mean (SD).
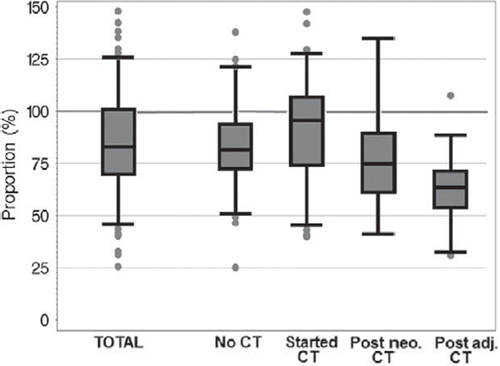
The median VO2peak values in our patient population were significantly lower than the expected cardiorespiratory values according to the age- and BMI-related prediction formula [Citation15] (). Patients post adjuvant or neo-adjuvant chemotherapy reached only a median of 63% or 75% of expected VO2peak, respectively. In contrast, patients who just started chemotherapy reached 96% of expected VO2peak.
Figure 2. Distribution of VO2peak, presented as percentage of expected values for healthy women with the same age and BMI. The boxes present the 1st and 3rd quartiles, the middle line the medians, and the Whisker-ends the 5th and 95th percentiles.
In ANCOVA models the categorical treatment variable was significantly associated with VO2peak (p < 0.01, ), when adjusted for influencing factors such as age (p < 0.01), BMI (p < 0.01), cycling (p = 0.03), and smoking before diagnosis (p = 0.07). Hereby, the adjusted mean VO2peak among patients who had just started chemotherapy did not differ from those with no chemotherapy; however, the adjusted means of those two groups were significantly higher compared to post adjuvant chemotherapy. The group which just started the chemotherapy and which had no chemotherapy had a more favorable VO2peak compared to those who completed treatment. Notably, the VO2peak was even more reduced among those who finished adjuvant chemotherapy compared to those prescribed neo-adjuvant chemotherapy. There was no significant association between the type of anti-cancer treatment and VT in the final adjusted model. The only significant determinant of VT was BMI (p < 0.01). However, treatment was found to be borderline significant (p = 0.057) in the model without adjustment for walking, cycling, and sport activities, with highest VT values among those who had started chemotherapy (data not shown).
Figure 3. Adjusted mean values of each treatment category regarding VO2peak, VT, and HR50. Least square means were calculated using analyses of covariance models adjusted for age, BMI, smoking before diagnosis, walking, cycling, and sports before diagnosis, sports during adolescence, use of beta-blocker, and pre-existing heart diseases. Differences between categories were tested using Tukey-Kramer tests and all statistically significant differences (p < 0.05) are indicated in the figures.
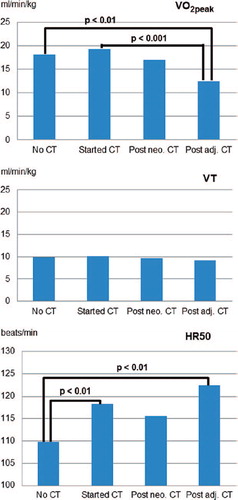
In contrast, HR50 was significantly associated with treatment (p < 0.01) with significantly lower adjusted mean resting HR in patients with no chemotherapy compared to patients during or post adjuvant chemotherapy (p < 0.01). The only other covariate that showed a borderline significant association with HR50 in the adjusted model was use of beta-blockers (p = 0.055) with lower HR among users. The adjusted models explained 46.1%, 31.8%, and 18.7% of the variance of VO2peak, VT, and HR50, respectively.
Receipt of chemotherapy was also associated with decreased Hb-level, especially after completion of adjuvant chemotherapy (p < 0.0001). However, Hb-level showed no significant association with VO2peak, VT, or HR50, or any association between treatment regimes. These fitness measures did not alter when the models were adjusted additionally for Hb. Therefore, Hb was not a significant mediator of the effects of chemotherapy on cardiorespiratory fitness.
Among patients who had just started adjuvant chemotherapy, 85% had received anthracyclines and only 39% had received taxanes. In contrast, nearly all patients’ post adjuvant or neo-adjuvant chemotherapy had received both, anthracyclines and taxanes, during the course of their chemotherapy (see ). Thus, the independent effects of those chemotherapeutic agents on VO2peak could not be determined. However, aforementioned treatment effect on VO2peak did not alter substantially when adjusting the model for taxane and anthracycline regimen. When investigating the subgroup of patients who had started adjuvant chemotherapy, the adjusted means of VO2peak were nearly identical for anthracycline use only, taxane use only, and combined therapy (data not shown). Similar results were found for the adjusted means of HR50. Adjustment for trastuzumab or hormone therapy was not found to alter the results.
Discussion
The present study of 222 non-metastatic breast cancer patients revealed alarmingly low cardiorespiratory fitness in breast cancer patients across the treatment continuum. The mean VO2peak among our sample population was 20.6 ± 6.7 ml/min/kg, compared to the expected mean VO2peak of 24.3 ± 5.5 ml/min/kg among healthy population of comparable age and BMI distribution [Citation15].
Cardiorespiratory fitness was significantly associated with type and stage of cancer treatment, with lower adjusted mean VO2peak among patients post adjuvant chemotherapy compared to patients with no chemotherapy or who had just started chemotherapy. On average, patients who had just started chemotherapy (i.e. had one or two cycles of chemotherapy) were capable of reaching 96% of the VO2peak level that is expected for their age- and BMI-category; however, among patients receiving post adjuvant chemotherapy (i.e. about 4 weeks after completion of chemotherapy), fitness levels were only 63% of the expected level.
Cardiopulmonary exercise testing (CPETs) have been universally utilized in several randomized exercise trials [Citation8,Citation18], however, there have been few systematic investigations of effects of cancer therapy on cardiorespiratory fitness among breast cancer patients. To our knowledge, there has been only one study comparing VO2peak at various points of chemotherapy treatment and compared with the expected capacity in healthy women. Our study expands on previous methods by assessing submaximal exercise measures in addition to peak measures. This enabled analyses of patients who could not perform maximal exercise testing until exhaustion, which is a limitation of exercise testing in an oncology setting.
The considerable reduction in VO2peak compared to healthy individuals is in accordance with the findings of Jones et al. [Citation18]. It therefore appears to be of good external validity, but still needs confirmation in future studies. Jones and colleagues [Citation18] found that breast cancer patients during chemotherapy reached 73% of age-expected capacity of healthy sedentary women with a mean VO2peak of 17.4 ± 4.3 ml/min/kg. This is consistent with our results, which revealed a mean VO2peak of 15.5 ± 4.8 ml/kg/min about four weeks post adjuvant chemotherapy. Several intervention trials demonstrated VO2peak measures in breast cancer patients or survivors ranging between 18 and 26 ml/min/kg [Citation8]. All published comparable studies that we know of, reported similar results to our findings [Citation18–22]. In Jones et al. [Citation18], marginally lower VO2peak values were described in partly metastatic breast cancer patients. Slightly higher values in other referenced studies [Citation19,Citation20,Citation22] might be attributable to a younger population; differences in the test method, namely higher VO2peak values, can be expected when using treadmill tests. The lower VO2peak values in Drouin et al. [Citation21] can be explained due to recruitment of only sedentary population. However, these results lack adjustment for age, BMI or other potentially influencing factors, and had large heterogeneity in the time since or end date of chemotherapy; therefore, these studies could not be properly compared to the results of the present study. Notably, the average VO2peak in patients post-chemotherapy showed similar impairment in cardiac capacity as that observed among 1052 women with a myocardial infarction (15.4 ± 4.0 ml/kg/min, measured about 14 weeks after the event) [Citation23]. The study population of these cardiac patients was of comparable age (58.5 ± 9.8 years) and BMI (26.7 ± 5.1 kg/m2) and followed a similar testing protocol on a cycle ergometer as the present study.
Interestingly, in contrast to the objective measures of cardiorespiratory function, our attending oncologists (without being aware of the CPET results) assessed the performance of the majority of patients as ECOG = 0, i.e. as fully active, able to carry on all pre-disease activities of daily living without restrictions. These results emphasize that impaired cardiorespiratory fitness is prevalent among breast cancer patients, and should be monitored and counteracted to avoid further negative consequences. Low exercise levels after breast cancer treatment should be avoided, because lack of exercise is an established predictor of mortality in both breast cancer patients and healthy women [Citation3,Citation4]. Furthermore, low cardiorespiratory fitness has been observed as significant determinant of dependence and functional limitations in daily living among older adults [Citation24].
Furthermore, the impact of cancer treatment on the complex oxygen cascade, which involves several components of the respiratory and cardiovascular systems, is only marginally understood [Citation6]. The inclusion of three different fitness parameters in our analysis provides further insight into the impact of chemotherapy on specific organs and functions within the cardiorespiratory system. VO2peak was chosen as a global parameter, which is influenced by oxygen uptake in the lung (ventilation and gas exchange), oxygen delivery (heart and blood), and peripheral oxygen extraction (metabolism on muscular level) [Citation5]. As VO2peak depends on maximal effort and not all patients are willing or able to spend maximal effort, submaximal parameters, such as VT and HR50, are considered. VT provides insight into energy metabolism (aerobic vs. anaerobic) in the muscle and is defined as the point during graded exercise at which ventilation increases disproportionately to O2 uptake. HR50 or at a given submaximal work load provides information regarding the heart rate response, an important contributor to exercise intolerance. Our multiple regression analyses revealed that chemotherapy treatment has a significant impact on VO2peak, independent of other influencing factors such as age, BMI, or previous regular aerobic exercise. Effects of chemotherapeutic agents on different organ components of the oxygen system (e.g. the respiratory, cardiac, blood, vascular, or skeletal muscle functions) have been observed or hypothesized, potentially contributing to impaired cardiorespiratory fitness [Citation6]. Our adjusted regression analyses showed that HR50 was significantly affected by chemotherapy treatment. One can speculate that this observation is related to an impaired oxygen transportation of the blood which is compensated by a higher heart rate. VT was not significantly associated with chemotherapy, suggesting that there is no substantial impairment in skeletal muscle function as a result of chemotherapy treatment. However, the direct effects of cancer therapy are not easy to distinguish from indirect effects of therapy, such as detraining or changes in body weight and composition as consequence of physical inactivity. Reduced physical activity may also be a potential consequence of poor cardiorespiratory fitness post-cancer therapy. Cancer-related fatigue, which often persists during and after anti-cancer therapy and lack of knowledge of the benefits of effects of exercise, might increase sedentary behavior. Among breast cancer patients, a significant reduction in physical activity during cancer therapy has been observed, with a large proportion of women remaining inactive or demonstrating reductions in physical activity level post-therapy compared to pre-diagnosis [Citation25]. To counteract this vicious cycle (i.e. low fitness leading to decreased activity leading to further decreased fitness), physicians and health professionals should encourage breast cancer patients to be physically active during therapy and to engage in exercise programs. There is strong evidence from randomized intervention studies that even strenuous exercise, including aerobic as well as resistance training, is feasible and can be safely performed in breast cancer patients during and after therapy [Citation26]. A meta-analysis of endurance exercise interventions in breast cancer patients reported an average 14.8% increase in VO2peak [Citation27]. A recent meta-analysis by Jones and colleagues reported similar cardiorespiratory fitness benefits among cancer patients participating in exercise programs [Citation28].
There are several limitations to this study. The investigated size of the treatment groups was unequal, because the studies (BEST and BEATE) were not primarily designed for these comparisons. Additionally, the cross-sectional design (i.e. using the baseline measurements only) limits causal inferences. Measurements of cardiorespiratory fitness are repeated at several time points in our intervention trials, therefore longitudinal investigations are possible in the future. A methodological issue is that our exercise protocol started at a relatively high load level (50 watts). VT was reached at an average of 47 watts, which is within the first exercise stage. The VT would be more easily identifiable and determined better if at least one complete exercise stage was achieved below the anticipated VT or by using a ramp incremental test protocol. Therefore the VT results must be interpreted with caution. Given the problems with VT, future studies should elucidate to importance of measuring VT in cancer patients by evaluating the suitability of different testing protocols to detect VT more precisely.
Strengths of our study include the use of the gold standard of cardiorespiratory fitness testing for the assessment of overall cardiorespiratory function. The additional investigation of submaximal measures, (i.e. VT and HR50) provides insight into the effects on muscular metabolic function and cardiocirculatory values. Submaximal parameters are independent of maximal effort. As 46 of 222 participants could not reach the criteria for maximal effort, the submaximal data still provided relevant information on the patients’ cardiorespiratory fitness. In the absence of maximal exertion results, this data can be used to advise patients on endurance training prescriptions. Furthermore, the present study utilized information from a relatively large patient group and assessed many cofactors, which enabled adjusted regression modeling. Various breast cancer treatment characteristics were explored. These subgroups were recruited under comparable conditions from the same population source resulting in reliable findings.
In conclusion, breast cancer patients had marked and significantly impaired cardiorespiratory function during and post-chemotherapy. The impact on VO2peak appears to accumulate over the course of chemotherapy, while HR was already impaired during the first chemotherapy cycles. However, there was no significant association between chemotherapy treatment and VT. Overall, our data suggest that chemotherapy may adversely affect cardiorespiratory fitness by negatively influencing the oxygen delivery system rather than impacting metabolic muscle function.
These findings underline the need of systematic exercise training to counteract the loss of cardiorespiratory fitness during the anti-cancer treatment in breast cancer patients. In conjunction with the recommendations of other studies [Citation29,Citation30], exercise therapy should become a substantial and integrative part of supportive oncology therapy, with the ultimate goal to mitigate treatment-related side effects and improve quality of life and survival after breast cancer.
Acknowledgements
The BEST-Study is funded by the Interdisciplinary Research Funding Program (intramural) of the National Center for Tumor Diseases (NCT), Heidelberg and supported by the Foundation “Stiftung Leben mit Krebs”. Dr. Wiskemann was personally supported by the Manfred-Lautenschlaeger-Foundation. “Stiftung Leben mit Krebs”. The authors thank the study participants who willingly spent their time to complete the study procedures, the breast cancer centers supporting the recruitment, Petra Armbrust, Tilla Ruf (PhD), Renate Schoenmakers, Sabine Wessels (PhD), Sandra Gollhofer, Brigitte Wiegand, Simone Stefaniszyn and Marina Sumic for study coordination and assistance and Werner Diehl for data management. We owe special thanks to Holger Hof (MD), Jan Oelmann (MD), Andrea Koffka (MD), Henrik Hauswald (MD), Ulrike Bussas (MD), and Simone Hummler (MD), for medical support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W. SEER cancer statistics review, 1975–2008, National Cancer Institute. Bethesda, MD. Available from: http://seercancergov/csr/1975_2008/ based on November 2010 SEER data submission. [posted on SEER web site 2011].
- Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res 2011;13:R64.
- Peel JB, Sui X, Adams SA, Hebert JR, Hardin JW, Blair SN. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med Sci Sports Exerc 2009; 41:742–8.
- Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr., et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996;276:205–10.
- American Thoracic S, American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–77.
- Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol 2012;9:288–96.
- Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol 2008;9:757–65.
- Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev 2013;9:CD010192.
- Potthoff K, Schmidt ME, Wiskemann J, Hof H, Klassen O, Habermann N, et al. Randomized controlled trial to evaluate the effects of progressive resistance training compared to progressive muscle relaxation in breast cancer patients undergoing adjuvant radiotherapy: The BEST study. BMC Cancer 2013;13:162.
- Schmidt ME, Wiskemann J, Krakowski-Roosen H, Knicker AJ, Habermann N, Schneeweiss A, et al. Progressive resistance versus relaxation training for breast cancer patients during adjuvant chemotherapy: Design and rationale of a randomized controlled trial (BEATE study). Contemp Clin Trial 2013;34:117–25.
- Howley ET, Bassett DR, Jr., Welch HG. Criteria for maximal oxygen uptake: Review and commentary. Med Sci Sport Exerc 1995;27:1292–301.
- Midgley AW, McNaughton LR, Polman R, Marchant D. Criteria for determination of maximal oxygen uptake: A brief critique and recommendations for future research. Sports Med 2007;37:1019–28.
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986;60:2020–7.
- Meyer T, Lucia A, Earnest CP, Kindermann W. A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters – theory and application. Int J Sport Med 2005;26(Suppl 1):S38–48.
- Koch B, Schaper C, Ittermann T, Spielhagen T, Dorr M, Volzke H, et al. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J 2009;33:389–97.
- Textor J, Hardt J, Knuppel S. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011;22:745.
- Midgley AW, Bentley DJ, Luttikholt H, McNaughton LR, Millet GP. Challenging a dogma of exercise physiology: Does an incremental exercise test for valid VO 2 max determination really need to last between 8 and 12 minutes?Sports Med 2008;38:441–7.
- Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 2012;30:2530–7.
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–404.
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of a randomized controlled trial. J Clin Oncol 2001; 19:657–65.
- Drouin JS, Armstrong H, Krause S, Orr J. Effects of aerobic exercise training on peak aerobic capacity, fatigue, and psychological factors during radiation for breast cancer. Rehabil Oncol 2005;23:11–7.
- Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs 2006;29:156–65.
- Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am College Cardiol 2003;42:2139–43.
- Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst 2006;98:521–9.
- Huy C, Schmidt ME, Vrieling A, Chang-Claude J, Steindorf K. Physical activity in a German breast cancer patient cohort: One-year trends and characteristics associated with change in activity level. Eur J Cancer 2012;48:297–304.
- Cheema B, Gaul CA, Lane K, Fiatarone Singh MA. Progressive resistance training in breast cancer: A systematic review of clinical trials. Breast Cancer Res Treat 2008;109: 9–26.
- Kim CJ, Kang DH, Park JW. A meta-analysis of aerobic exercise interventions for women with breast cancer. Western J Nurs Res 2009;31:437–61.
- Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: A meta- analysis. Oncologist 2011;16:112–20.
- Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncol 2013;52:195–215.
- Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: A meta-analysis. Med Sci Sport Exerc 2013;45:2080–90.