To the Editor,
The current standard of care for patients with newly diagnosed glioblastoma consists of maximal safe surgical resection, followed by radiotherapy with concomitant and adjuvant chemotherapy with temozolomide for people younger than 65 years [Citation1] and radiotherapy or chemotherapy with temozolomide alone for elderly patients depending on the methylation status of the O6-methylguanine DNA methyltransferase (MGMT) gene promoter [Citation2,Citation3]. Nevertheless, the tumor recurs in virtually all patients, and there is no agreed standard of care for progressive disease [Citation4]. Re-challenge with temozolomide and the administration of nitrosoureas are among the most frequently used options, particularly in patients who are still in overall good condition [Citation5]. In 2009, bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), was approved by the FDA but not in many other countries including those of the European Union for patients with recurrent or progressive glioblastoma [Citation6,Citation7]. The combination of bevacizumab with other cytotoxic agents, such as irinotecan or temsirolimus, has not provided signals of activity over those achieved with bevacizumab alone [Citation7–11]. Preliminary data suggest a putative benefit for the combination of bevacizumab and lomustine in patients with glioblastoma progressing after temozolomide-based chemoradiation [Citation12]. Upon further tumor progression on bevacizumab therapy, additional treatment options are urgently needed, but the activity of all agents tested so far was low. Whether bevacizumab should be stopped upon tumor progression or continued while adding another drug has also remained a matter of debate. While immediate discontinuation may result in progressive clinical deterioration due to withdrawal of the anti-edematous activity [Citation13], the continued application may be associated with additional toxicity. Here we report our institutional experience with the addition of lomustine in patients with recurrent glioblastoma who progressed on bevacizumab monotherapy.
Patients and methods
We retrospectively reviewed the tumor board proceedings from 2010 to 2013 for patients with recurrent glioblastoma treated with bevacizumab (10 mg/kg qow). Lomustine (CCNU, 90–110 mg/m2 q 6 weeks) was added to the regimen upon further tumor progression while bevacizumab was continued. Magnetic resonance imaging (MRI) was performed in 8–12 week intervals or upon clinical deterioration. Radiographic progression was defined by an increase of at least 25% of contrast enhancing tumor in T1 MRI scans or non-enhancing tumor in T2 MRI scans according to Response Assessment in Neuro-Oncology (RANO) criteria. Progression-free survival (PFS) was calculated according to the Kaplan-Meier method from the date of previous progression on bevacizumab monotherapy until the date of further progression on salvage bevacizumab plus lomustine therapy confirmed by MRI. Survival was calculated from the date of progression on treatment with bevacizumab monotherapy to the date of death. The total overall survival was calculated from the date of surgery to the date of death. Spearman's rank correlation analysis was performed for time on bevacizumab monotherapy with survival after progression under bevacizumab monotherapy. No approval of the institutional ethics committee was needed for this retrospective anonymized analysis according to the local regulations.
Results
We identified 20 glioblastoma patients who had been treated with bevacizumab at tumor relapse and add-on lomustine escalation upon further progression. summarizes essential patient characteristics including the applied treatment lines. The median age at diagnosis was 52.5 years (range 36–73 years) with a preponderance of males (14 males, 6 women). Eighteen patients had received radiotherapy with concomitant and adjuvant temozolomide and two elderly patients had received radiotherapy or chemotherapy alone as first-line treatment depending on the MGMT promoter methylation status [Citation4]. In 15 patients, bevacizumab was introduced at first recurrence/progression, and five patients received bevacizumab after re-exposure to a dose-intensified temozolomide regimen at initial tumor progression. Upon tumor progression on bevacizumab, all patients were continued on bevacizumab and treatment escalation was accomplished by the addition of lomustine at second (75%) or third (25%) tumor recurrence.
Table I. Patient characteristics.
The addition of lomustine was overall well tolerated. However, hematological toxicity was common. Twelve patients (60%) developed CTCAE grade 3–4 hematotoxicity. Of these, five patients (25%) had grade 3–4 leukopenia and neutropenia, four patients (20%) suffered from grade 3 thrombocytopenia and three (15%) patients developed grade 3–4 lymphopenia. One patient received recombinant granulocyte-colony stimulating factor (G-CSF, filgrastim) because of prolonged neutropenia resulting in a delay of two weeks of lomustine continuation and a lomustine dose reduction of 25%.
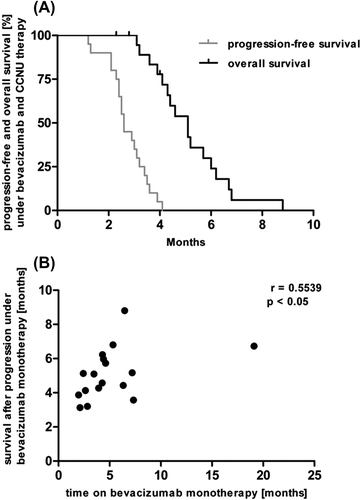
The median PFS for patients on bevacizumab monotherapy at first or second tumor progression was 4.3 months. All patients received lomustine in combination with bevacizumab after progression on bevacizumab monotherapy. The median PFS (mPFS) after escalation of bevacizumab therapy with lomustine was 2.6 months. PFS at 6 months after the initiation of bevacizumab/lomustine was 0%. The median OS (mOS) after initiation of the bevacizumab/lomustine regimen was 5.1 months (). There was a statistically significant correlation between the time on bevacizumab monotherapy with survival upon tumor progression (r = 0.5539 and p < 0.05 according to Spearman's correlation analysis) (). The mOS from initial diagnosis was 18.6 months.
Figure 1 (A) Progression-free and overall survival of 20 patients with second or third progression of glioblastoma treated with bevacizumab and lomustine upon progression on bevacizumab monotherapy (OS with 3 censored cases because of unknown time of death (n = 2) or still alive (n = 1) at the time of closure of the database). (B) Correlation between time on bevacizumab monotherapy and survival following progression (p < 0.05; Spearman's correlation).
Discussion
Controversy remains on optimal treatment of patients with recurrent glioblastoma. Although bevacizumab is commonly used, timing of administration and optimal patient management upon further progression remain undefined. Initial reports of high radiological response rates in patients with recurrent glioblastoma treated with bevacizumab led to approval in the US and Switzerland, but not the European Union [Citation6,Citation7]. Currently, there are no data to support the combination of bevacizumab with another agent in this indication [Citation5]. Furthermore, there is no consensus on how patients who experience further disease progression upon bevacizumab salvage therapy should be treated [Citation9,Citation10]. Lomustine has been used for a long time for the treatment of patients with recurrent glioblastoma and has also been frequently administered within clinical trials as the standard treatment arm [Citation14,Citation15]. Here we report that the addition of lomustine after progression on bevacizumab monotherapy as third- or fourth-line therapy fails to induce relevant disease stabilization, but is associated with hematological toxicity in 60% of these heavily pretreated patients. Our results are consistent with previous studies that had failed to show any benefit from therapy escalation with other drugs following disease progression under a bevacizumab containing regimen [Citation7,Citation9–11].
In contrast to its questionable effect on overall survival, there are convincing data demonstrating an anti-edematous activity of bevacizumab [Citation13]. Although the decrease of the interstitial pressure should allow for a better distribution of cytotoxic agents into the tumor, the normalization of the blood-brain barrier function by bevacizumab may hamper the penetration of cytotoxic agents administered after tumor progression on bevacizumab therapy [Citation16,Citation17]. Thus, prior treatment with bevacizumab may preclude benefit from other agents administered afterwards. This mechanism has been shown in preclinical and clinical studies combining anti-angiogenic compounds with alkylating agents [Citation18,Citation19]. Other reports demonstrated mOS of 5.9 months for bevacizumab continuation beyond initial progression on bevacizumab in patients with recurrent glioblastoma [Citation20] which is similar to the results obtained with lomustine in our study. In patients with anaplastic gliomas, continuation of bevacizumab beyond progression did not result in promising survival data [Citation21]. A retrospective series analyzing the sequence of bevacizumab and lomustine revealed similar outcomes independent of which agent was used first. However, bevacizumab resulted in a longer PFS when it was administered first [Citation22]. Our data suggest a positive correlation for the time on bevacizumab monotherapy with subsequent survival (). A randomized phase II study compared bevacizumab or lomustine monotherapy with the combination of both agents in patients with first recurrence of glioblastoma. Here, a higher activity of the combined treatment approach yielding PFS-6 of 41% was observed compared to either treatment alone [Citation12]. Based on these findings, the EORTC 26101 trial has been amended and will be continued as a phase III study comparing lomustine monotherapy with the combination of bevacizumab and lomustine for glioblastoma patients with first tumor recurrence (NCT01290939). Only the results of this and similar trials will help to define the role of both compounds in the setting of recurrent glioblastoma.
Acknowledgments
We thank Dr. Andreas Keller for statistical advice.
Declaration of interest: Katharina Seystahl has received honoraria for advisory board participation from Roche. Roger Stupp has served on advisory boards for Roche/Genentech, MSD and EMD-Serono/Merck. Michael Weller has received research grants from Bayer, Isarna, MSD, Merck Serono and Roche and honoraria for lectures or advisory board participation from Isarna, Magforce, MSD, Merck Serono, Pfizer, Roche and Teva. Patrick Roth has received honoraria from MSD, Roche and Molecular Partners for advisory board participation. Michaela Tonder, Günter Eisele, Tobias Weiss, Silvia Hofer and Antonios Valavanis report no disclosures.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med 2005;352:987–96.
- Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916–26.
- Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13:707–15.
- Weller M, Van den Bent M, Hopkins PA, Tonn JC, Stupp R, Falini R, et al. EANO guideline on the diagnosis and treatment of malignant glioma. Lancet Oncol (in press).
- Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma – are we there yet? Neuro Oncol 2013;15:4–27.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009; 27:4733–40.
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740–5.
- Lassen U, Sorensen M, Gaziel TB, Hasselbalch B, Poulsen HS. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res 2013;33:1657–60.
- Reardon DA, Desjardins A, Peters K, Gururangan S, Sampson J, Rich JN, et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol 2011;103:371–9.
- Reardon DA, Desjardins A, Peters KB, Vredenburgh JJ, Gururangan S, Sampson JH, et al. Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer 2011;117:5351–8.
- Quant EC, Norden AD, Drappatz J, Muzikansky A, Doherty L, Lafrankie D, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol 2009;11:550–5.
- Taal W, Oosterkamp HM, Walenkamp AME, Beerepoot LV, Hanse M, Buter J, et al. A randomized phase II study of bevacizumab versus bevacizumab plus lomustine versus lomustine single agent in recurrent glioblastoma: The Dutch BELOB study. ASCO Meeting Abstracts 2013;31:2001.
- Roth P, Regli L, Tonder M, Weller M. Tumor-associated edema in brain cancer patients: Pathogenesis and management. Expert Rev Anticancer Ther 2013;13:1319–25.
- Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. Expert Rev Anticancer Ther 2010;28:1168–74.
- Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 2013;31:3212–8.
- Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology 2011;76:87–93.
- Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ, et al. Concerns about anti- angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer 2009;9:444.
- Claes A, Wesseling P, Jeuken J, Maass C, Heerschap A, Leenders WP. Antiangiogenic compounds interfere with chemotherapy of brain tumors due to vessel normalization. Mol Cancer Therapeut 2008;7:71–8.
- Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: Implications for scheduling of anti-angiogenic drugs. Cancer Cell 2012;21:82–91.
- Reardon DA, Herndon JE, 2nd, Peters KB, Desjardins A, Coan A, Lou E, et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer 2012;107:1481–7.
- Reardon DA, Herndon JE, 2nd, Peters K, Desjardins A, Coan A, Lou E, et al. Outcome after bevacizumab clinical trial therapy among recurrent grade III malignant glioma patients. J Neurooncol 2012;107:213–21.
- Wiestler B, Radbruch A, Osswald M, Combs SE, Jungk C, Winkler F, et al. Towards optimizing the sequence of bevacizumab and nitrosoureas in recurrent malignant glioma. J Neurooncol 2014;117:85–92.
