Abstract
Background. Inhibitors of the mammalian target of rapamycin (mTOR) are currently approved for the treatment of several cancers, and their use is associated with serious rash, which affects patient's quality of life and leads to undesirable dose reductions or interruptions. A meta-analysis of randomized controlled trials (RCTs) was performed to determine the overall risk of developing high-grade rash with mTOR inhibitors in cancer patients.
Methods. We searched the PubMed database and abstracts presented at the American Society of Clinical Oncology (ASCO) meetings up to December 2013 for relevant studies. Eligible studies included RCTs in which everolimus or temsirolimus was compared to controls in cancer patients. The summary incidence, relative risk (RR), and 95% confidence intervals (CI) were calculated using a random- or fixed-effects model depending on the heterogeneity of the included trials.
Results. A total of 11 RCTs with 4752 patients (mTORs: 2725, controls: 2027) with a variety of solid tumors were included in the analysis. The incidences of all-grade (grade 1–4) and high-grade rash (grade 3–4) were 27.3% (95% CI 21.0–34.7%) and 1.0% (95% CI 0.6–1.4%), respectively. In comparison with controls, mTOR inhibitors significantly increased the risk for developing all-grade rash (RR = 3.55, 95% CI 3.0–4.20, p < 0.001) and high-grade rash (RR = 4.25, 95% CI 1.63–11.10, p = 0.003). The increased risk of high-grade rash did not vary significantly among different tumors (p = 0.91). There was no significant difference between everolimus and temsirolimus (p = 0.60). There was also no significant difference between mTOR inhibitors alone and in combination with other agents (p = 0.57).
Conclusions. Everolimus and temsirolimus significantly increased the risk of high-grade rash in cancer patients. Early recognition and appropriate treatment is recommended.
The mammalian target of rapamycin (mTOR) plays an essential role in multiple signaling cascades as a downstream effector of the PI3-k/Akt/mTOR pathway [Citation1,Citation2]. Unregulated expression of mTOR has been implicated in the pathogenesis of various solid tumor and hematological malignancies. On a cellular level, constitutive activation leads to excessive production of proteins that has been implicated in tumorigenesis, such as cyclin D and hypoxia inducible factors (HIFs) [Citation2]. Due to this, mTOR has become a principal target for treatment of cancer in clinical practice.
The FDA approved the first mTOR inhibitor, sirolimus in 1999, for the prevention of rejection in transplant, due to its immunosuppressive effects [Citation1]. This was followed by the development of the first generation mTOR inhibitors (everolimus, temsirolimus, and ridaforolimus) that unlike sirolimus, are chemically stable for required dosing in cancer patients [Citation3]. First generation mTOR inhibitors act via inhibition of the rapamycin sensitive mTORC1 protein complex. Currently everolimus is approved for the treatment of advanced renal cell carcinoma (RCC) after failure with sunitinib or sorafenib, progressive pancreatic neuroendocrine tumor (PNET), advanced hormone receptor positive HER2-negative breast cancer in combination with exemestane, and sub-ependymal giant cell astrocytoma (SEGA) in both pediatric and adult patients. In 2007, the FDA approved the intravenous form of temsirolimus for the treatment of advanced renal cell carcinoma with poor prognosis.
Common adverse effects of mTOR inhibitors include rash, stomatitis, hyperlipidemia, hyperglycemia, thrombocytopenia, anemia, and fatigue [Citation2,Citation4]. Rash has frequently been observed in clinical trials evaluating the toxicity of everolimus and temsirolimus. Serious rash secondary to mTOR inhibitor therapy affects the quality of life and results in undesirable dose reduction and drug interruption or discontinuation. We conducted a meta-analysis of randomized controlled trials (RCTs) to determine the overall incidence and relative risk of developing high-grade rash with mTOR inhibitors.
Methods
Data source
The PubMed database (www.pubmed.gov) was independently searched from 1 January 1998 to 31 December 2013 using the key words “everolimus” and “temsirolimus.” The search was limited to clinical trials in cancer patients. Clinical trials that studied the use of mTOR inhibitors for alternate indications such as immunosuppression in transplant patients were excluded. In addition, we searched abstracts presented at the American Society of Clinical Oncology (ASCO) conferences from 2004 to 2013 using the key words “everolimus” “temsirolimus” and “rash.” ASCO posters were reviewed for complete adverse event information regarding rash. Each publication was reviewed to ensure that the most recent and up to date version was identified for duplicate publications of a clinical trial.
Study selection
Non-randomized clinical trials were excluded in our analysis. Phase I trials were excluded in the meta-analysis due to multiple dose levels. For inclusion, all selected studies were phase II and III prospective randomized controlled clinical trials in which the mTOR inhibitors were compared to placebo or other drugs. RCTs of everolimus and temsirolimus were included in our analysis. We did not incorporate RCTs that evaluated the efficacy and safety of ridaforolimus. Included trials were required to provide the number and/or percentage of patients with all-grade (1–4) and high-grade (3–4) adverse events based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE). All included trials were required to label “rash” for reported adverse effects. In an effort to incorporate all available relevant clinical trials with complete rash incidence data, trials included in our meta-analysis were allowed to administer everolimus and temsirolimus at other doses as long as it was a RCT. For everolimus, trials that administered an approved dose of 10 mg by mouth once a day or titrated to achieve a therapeutic blood trough concentration were included in the analysis. Also included were trials that administered temsirolimus at the US FDA approved dose of 25 mg infused over a 30–60 minute period once a week, or at the dose of 30 mg by mouth (5 days every 2 weeks), that has shown tolerability and efficacy in a randomized phase II trial [Citation5]. To assess study quality, the seven-item scale (score 0–5) Jadad Score was used for each included clinical trial.
Clinical end points
Clinical end points were extracted from the safety profile in each trial. Included trials described the incidence of rash as all-grade (grades 1–5) and high-grade (grade ≥ 3). Both all-grade rash and high-grade rash were recorded for analysis based on NCI-CTCAE versions 2.0 or 3.0. For version 2.0: grade 1, macular or papular eruption or erythema without associated symptoms, grade 2, macular or papular eruption or erythema with pruritus or other associated symptoms covering < 50% of body surface or localized desquamation or other lesions covering < 50% of body surface area, grade 3, symptomatic generalized erythroderma or macular, papular or vesicular eruption or desquamation covering ≥ 50% of body surface area, grade 4, generalized exfoliative dermatitis or ulcerative dermatitis, grade 5, death. For NCI-CTCAE version 3.0: grade 1, macular or papular eruption or erythema without associated symptoms, grade 2, macular or papular eruption or erythema with pruritus or other associated symptoms covering < 50% of body surface or localized desquamation or other lesions covering < 50% of body surface area, grade 3, severe, generalized erythroderma or macular, papular or vesicular eruption or desquamation covering ≥ 50% of body surface area, grade 4, generalized exfoliative dermatitis or bullous dermatitis, grade 5, death.
Statistical analysis
All statistical analyses were performed using version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, NJ, USA). The number of patients with rash (all-grade and high-grade) and the number of patients receiving everolimus or temsirolimus were extracted from included clinical trials. Additional data collected included dose of mTOR inhibitor, dose of comparator, specific cancer being studied, total cumulative all grade toxicity of rash, cumulative high-grade toxicity, and percentage of discontinuation secondary to dermatological toxicity. Collected data was entered into a Microsoft Excel sheet. For each study the proportion of patients with rash was calculated and the 95% confidence interval (CI) was derived. As all included clinical trials were designed to have a control arm, the relative risk of rash among patients assigned to everolimus or temsirolimus was calculated and compared to patients assigned to the control arm. For meta-analysis, both fixed-effects (weighted with inverse variance) and random-effects model were considered. Prior to the meta-analysis, Cochran's Q statistic was calculated to assess the heterogeneity among the proportions among the included trials. For a p-value of < 0.1, the assumption of homogeneity was considered invalid. If invalid the random-effects model was used. If the assumption of homogeneity as valid both the fixed-effects and random-effects model results were reported. We used the Begg's and Egger's tests to determine the presence of publication bias regarding primary endpoint (relative risk of all-grade and high-grade rash). A two-tailed p-value of < 0.05 was considered to be statistically significant.
Results
Search results
Our literature search generated a total of 591 potentially relevant studies of everolimus and temsirolimus. From these studies a total of 34 RCTs were identified, where 23 of them where excluded either due to incomplete data regarding the incidence of rash or suboptimal trial design where both treatment and control groups received mTOR therapy (). Overall, a total of 11 RCTs were included in our meta-analysis (everolimus = 9, temsirolimus = 2) [Citation6–16] (). These RCTs included both phase II (n = 2) and phase III trials (n = 3). The most recent publications of the included RCTs were analyzed. For example, the final version of the RECORD-1 trial, evaluating everolimus in metastatic renal cell carcinoma was selected in our meta-analysis [Citation9]. In an independent review, the Jadad Score was calculated for each included trial. Nine studies were given the highest Jadad score of 5, and two trials were given a score of 3, based on the seven-item scale ().
Figure 1. Selection of randomized controlled trials (RCTs) included in the meta-analysis. mTOR, mammalian target of rapamycin; RCTs, randomized controlled trials.
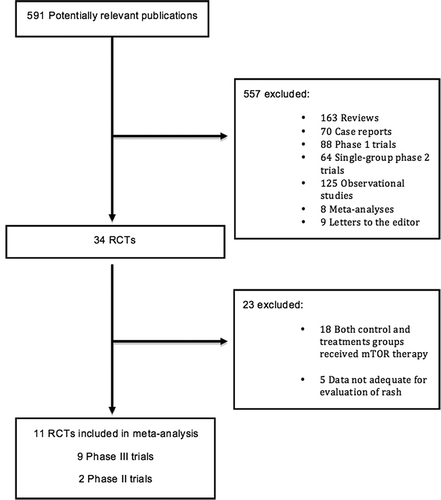
Table I. Characteristics of randomized controlled trials included in the meta-analysis.
Patients
A total of 4752 patients from 11 RCTs were available for analysis. From these patients, 997 received monotherapy with the everolimus FDA approved dose of 10 mg by mouth once a day with or without best supportive care (BSC) [Citation6–9]. In one trial, everolimus was administered in oral form based on body surface area (4.5 mg/m2 once a day) titrated to blood trough concentrations in 78 patients [Citation10]. Everolimus combination therapy with exemestane was given to 485 patients [Citation11]. Everolimus was administered with tamoxifen in 54 patients [Citation12]. Dual therapy with everolimus and letrozole was given to 137 patients [Citation13]. A total of 216 patients received everolimus with octreotide [Citation24]. Underlying malignancies treated with everolimus included angiomyolipoma [Citation7], gastric cancer [Citation8], renal cell carcinoma [Citation9], giant cell astrocytoma [Citation10], PNET (two trials) [Citation6,Citation14], and breast cancer (three trials) [Citation11–13].
In one of the included RCTs that evaluated temsirolimus for advanced renal cell carcinoma, a total of 208 patients received monotherapy with temsirolimus and 210 patients received temsirolimus with interferon [Citation15]. In a phase III trial for post-menopausal women with locally advanced or metastatic breast cancer, a regimen of temsirolimus 30 mg by mouth five days a week every two weeks with daily letrozole was given to 550 patients [Citation16].
Incidence of all-grade rash
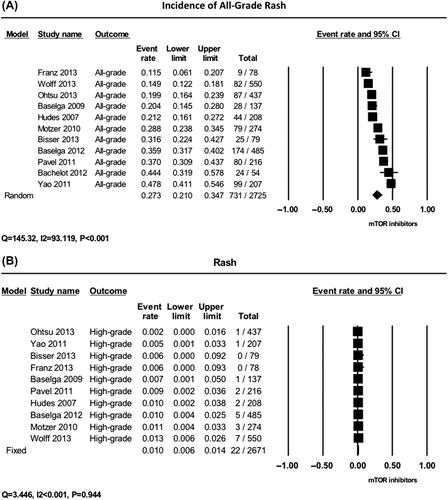
Data for all-grade rash included a total of 2725 patients, 1967 treated with everolimus and 758 treated with temsirolimus. Incidence of all-grade rash ranged from 11.5% to 47.8% with the lowest incidence in a phase III trial in patients with SEGA associated with tuberous sclerosis [Citation10], and the highest incidence in a phase III in patients with advanced PNET [Citation6]. The summary incidence of all-grade rash from the 11 RCTs in the meta-analysis was 27.3% (95% CI 21.0–34.7%), according to the random-effects model (Heterogeneity test: Q = 145.32, I2 = 93.119, p < 0.001) ().
Figure 2. Incidence of all-grade and high-grade rash to mTOR inhibitors. The summary incidences of all-grade (A) and high-grade (B) rash are calculated using the random-effects and fixed effects models, respectively. The incidence rate and 95% confidence interval (CI) for each trial, and the final combined results are demonstrated numerically on the left and graphically as a forest plot on the right. For individual trials: filled in square, incidence; lines, 95% confidence interval; diamond plot, overall results of the included trials.
Incidence of high-grade rash
High-grade skin rash is associated with morbidity, and results in dose reduction or discontinuation. Overall, 2725 patients with available data on high-grade rash were available for analysis. Incidence of high-grade rash ranged from 0.2% to 1.3% with the lowest incidence in a phase III in patients with advanced gastric cancer [Citation8], and the highest incidence in phase III trial in patients with locally invasive or metastatic breast cancer [Citation16]. Using a fixed-effects model (), the summary incidence of high-grade rash was 1.0% (95% CI 0.6–1.4%) (Heterogeneity test: Q = 3.446, I2 < 0.001, p = 0.94).
Relative risk of rash
Relative risk (RR) was calculated to determine the particular contribution of mTOR inhibitors to the development of rash by eliminating the influence of confounding factors, such as underlying malignancy, co-morbidities, and treatment with other medications. In comparison to controls, mTOR inhibitors significantly increased the risk for developing all-grade rash (RR = 3.55, 95% CI 3.0–4.20, p < 0.001) (). In addition, mTOR inhibitors significantly increased the risk of high-grade rash (RR = 4.25, 95% CI 1.63–11.10, p = 0.003) when compared to controls.
Figure 3. Relative risk (RR) of all-grade and high-grade rash to mTOR inhibitors. RRs for all-grade (A) and high-grade (B) rash were calculated using the fixed-effects model. The RR and 95% confidence interval (CI) for each trial and the final combined results are demonstrated numerically on the left and graphically as a forest plot on the right. For individual trials: filled in square, incidence; lines, 95% confidence interval; diamond plot, overall results of the included trials.
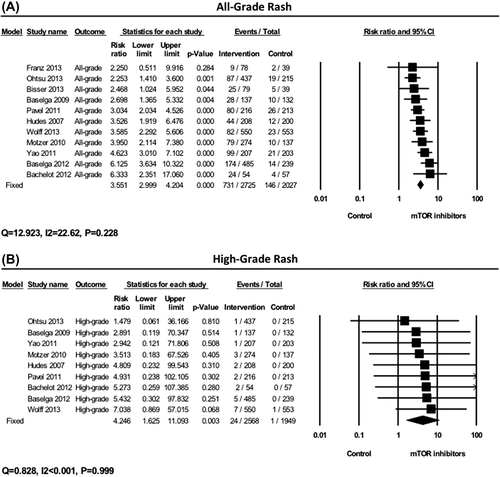
We also performed subgroup analysis for the RR of high-grade rash. In comparison to controls, everolimus significantly increased the risk of developing high-grade rash (RR = 3.57, 95% CI 1.12–11.36, p = 0.03). Also, temsirolimus significantly increased the risk of developing high-grade rash (RR = 6.22, 95% CI 1.11–34.81, p = 0.037). However, no significant difference between everolimus and temsirolimus was seen (p = 0.60). Our analysis did not show any significant variation between different tumor types (p = 0.91). Compared to controls, mTOR inhibitor did not significantly increase the risk of developing high-grade rash in PNET (RR = 3.86, 95% CI 0.43–34.80, p = 0.23), gastric cancer (RR = 1.48, 95% CI 0.061–36.17, p = 0.81), and RCC (RR = 4.09, 95% CI 0.49–33.97, p = 0.19). However, compared to controls, mTOR inhibitors significantly increased the risk of high-grade rash in breast cancer (RR = 5.37, 95% CI 1.41–20.54, p = 0.01). There was no significant difference between mTOR inhibitors alone and their combination with other agents (p = 0.57).
Publication bias
No publication bias was detected for and the primary endpoint RR of high-grade rash (Begg's test: p = 0.37; Egger's test: p = 0.92).
Discussion
Our meta-analysis has shown a statistically significant increased risk for developing all-grade and high-grade rash in cancer patients treated with mTOR inhibitors. The summary incidences of all-grade and high-grade rash are 27.3% (95% CI 21.0–34.7%) and 1.0% (95% CI 0.6–1.4%), respectively. A significantly increased risk for all-grade rash (RR = 3.55, 95% CI 3.0–4.20, p < 0.001) and high-grade rash (RR = 4.25, 95% CI 1.63–11.10, p = 0.003) was also demonstrated. Compared to controls, mTOR inhibitors significantly increased the risk of high-grade rash in breast cancer (RR = 5.37, 95% CI 1.41–20.54, p = 0.01). No significantly increased risk of high-grade rash was seen in other tumor types. The increasing widespread use of mTOR inhibitors as targeted therapy for various tumors emphasizes the importance of our results. Dermatologic toxicity from novel targeted anticancer agents, such as the mTOR inhibitors, can have a substantial impact on both the physical and psychosocial well-being of patients [Citation17]. For maximum clinical benefit both everolimus and temsirolimus require indefinite use, but rash-related drug discontinuation or sub-optimal dosing can impede this.
A systematic review and meta-analysis of the risk of rash with everolimus showed a statistically significant increased risk of all-grade rash (RR = 3.85, 95% CI 2.47–6.01, p < 0.001), but the RR of high-grade rash (RR = 3.0, 95% CI 0.63–14.19, p = 0.17) was not statistically significant [Citation18]. Temsirolimus individually also has been evaluated in a systematic review and meta-analysis of the risk of rash with its use [Citation19]. The meta-analysis results showed a significantly increased risk for all-grade rash (RR: 7.6, 95% CI 4.4–13.3), however the risk for high-grade rash (RR: 13.70, 95% CI 0.82–227.50, p = 0.07) did not reach statistical significance [Citation19]. Interestingly, our meta-analysis is the first to show a statistically significant increased risk for high-grade rash with mTOR inhibitors. This may be due to the inclusion of non-randomized clinical trials in prior meta-analyses [Citation18,Citation19]. In addition, our analysis is unique by investigating the risk of high-grade rash based on tumor type. Unlike all other tumors types, a significantly increased risk of high-grade rash was seen in breast cancer.
Both everolimus and temsirolimus are associated with various dermatological adverse effects such as rash, stomatitis, acne, hair changes, pruritus, and nail changes [Citation19]. Rash is one of the most common overall adverse effects with mTOR therapy. Onset of rash secondary to mTOR inhibitors is usually within the first month of initiation, where it can occur as early as the first week [Citation20,Citation21]. At onset rash usually appears as macules and papules with pustules on an erythematous base with centripetal spread [Citation18]. Size of rash usually ranges from 5 to 10 cm in diameter [Citation20,Citation21]. While a maculopapular rash is the most common presentation, acneiform and eczematoid variants have also been seen with mTOR inhibitor therapy [Citation21]. Distribution of the rash usually occurs at areas prevalent with sebaceous glands [Citation18]. Commonly affected areas include the scalp, face, neck, trunk, and extremities [Citation21,Citation22]. The rash can also present as an eczematoid eruption on the antecubital and popliteal regions [Citation17,Citation21].
The underlying etiology of rashes causes by mTOR inhibitors is currently unknown. It is hypothesized that cutaneous toxicity may arise from mTOR inhibition induced inhibitory effects on signaling pathways that regulate cell growth and repair [Citation22]. The most common documented reaction pattern seen in skin biopsies of rashes secondary to mTOR inhibitors is a mixed spongiotic interface and perivascular dermatitis, with mixed-cell infiltrates in the epidermis and dermis, characteristic of a drug reaction [Citation21,Citation22].
Early recognition and appropriate therapy is a key for the optimal management of rash secondary to mTOR inhibitors. Specific treatments for mTOR-induced rash should be based on the clinical phenotype of a patient [Citation21]. With prompt recognition, treatments such as topical moisturizers and powders may be effective for low-grade rashes (grade 1–2] [Citation19]. The preferred initial treatment of low-grade papulopustular rashes treatment includes topical low-moderate strength steroids (fluocinonide, clobetasol) and topical antibiotics (clindamycin) [Citation21,Citation23]. For grade 1 maculopapular rashes, topical low-moderate strength steroids are also used [Citation21]. For grade 3 and intolerable grade 2 rashes, oral antibiotics (e.g. doxycycline, minocycline) are recommended for duration of 2–4 weeks [Citation21]. Rash can be also be associated with pruritus. Treatment options for symptom amelioration include anti-histamines (e.g. hydroxyzine, diphenhydramine), topical corticosteroids, and antipruritic lotions [Citation19,Citation21]. Finally, patients should be educated about preventive measures, which include use of non-alcohol containing moisturizers, excessive sun exposure avoidance, sunscreen, and showering with a mild soap [Citation24].
All of the included trials in the meta-analysis except for one [Citation9] used NCI-CTCAE version 3.0 to assess and grade adverse events. As many of the currently used targeted cancer agents were unavailable at the time the version 3.0 was published, the accuracy for reporting of the type and grade of these specific dermatological adverse effects is questionable [Citation25]. Version 4.0, the most recent iteration of the NCI-CTCAE, has made some notable changes to the grading of maculopapular rashes, the most common type of rash seen with mTOR inhibitors. In version 4.0 of CTCAE, maculopapular rash is in its own category. Grades 1–3 are distinguished by the percentage of body surface area (BSA) involved (grade 1 < 10% BSA, grade 2 10–30% BSA, grade 3 > 30% BSA), and grades 2 and 3 also incorporate impact on activities on daily living (ADL) [Citation25]. Of note, version 4.0 of CTCAE does not include grades 4 and 5 because of the common perception that maculopapular rash does not lead to a life-threatening condition [Citation25]. This is consistent with our analysis of the included RCTs, where the overwhelming majority of high-grade rash events were grade 3 as opposed to grades 4 and 5. These necessary changes should ameliorate underreporting and inconsistent grading of a distinctive dermatologic event [Citation25]. It will be interesting to see how incorporation of version 4.0 in future randomized controlled trials will effect the grading of rash adverse effects and decision-making for dose reductions and interruptions.
This meta-analysis has several limitations. Results may be limited by the accuracy of individual RCTs to correctly assess the grade of severity of rash based on NCI-CTCAE version 3.0. This may have erroneously increased or decreased the incidence of all-grade and high-grade rash. The majority of included RCTs evaluated everolimus (n = 9), where only two temsirolimus trials were included in the final analysis. However, no significant difference was seen between everolimus and temsirolimus (p = 0.91). Another limitation was the inclusion of RCTs that did not administer mTOR inhibitors at approved doses. For everolimus, one trial dosed everolimus based on BSA, as opposed to the conventional oral 10 mg daily dosing regimen [Citation10]. Instead of the US FDA approved dose of 25 mg infusion on a weekly basis, temsirolimus was given orally (30 mg) in one trial [Citation16]. The oral dose showed similar risk of high-grade rash according to our analysis, and, has shown tolerability and efficacy in a randomized phase II trial [Citation5]. Also, our analysis did not include RCTs of the first-generation mTOR inhibitor, ridaforolimus. Future studies evaluating the risk of rash with ridaforolimus in different malignancies such as sarcoma will be of great interest.
In conclusion, our meta-analysis of RCTs has demonstrated a significantly increased risk for high-grade rash with mTOR inhibitors in cancer patients. This emphasizes that cancer patients treated with mTOR inhibitors should be aware of the common occurrence of rash and physicians should be cognizant of appropriate management strategies. Further research is necessary to clarify risk factors for developing mTOR inhibitor-induced high-grade rash. We hope that implementation of version 4.0 of the CTCAE in future clinical trials will improve reporting and consistency of grading for rash adverse events.
Declaration of interest: Dr. Wu is a speaker for Novartis and Pfizer. The authors alone are responsible for the content and writing of the paper.
References
- Dabney R, Devine R, Sein N, George B. New agents in renal cell carcinoma. Target Oncol Epub 2013 Nov 16.
- Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: Development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol 2009;2:45.
- Khokhar NZ, Altman JK, Platanias LC. Emerging roles for mammalian target of rapamycin inhibitors in the treatment of solid tumors and hematological malignancies. Curr Opinion Oncol 2011;23:578–86.
- Martins F, de Oliveira MA, Wang Q, Sonis S, Gallottini M, George S, et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol 2013;49:293–8.
- Carpenter JT, Roche H, Campone M, Colomer R, Jagiello-Gruszfeld A, Moore L, et al. Randomized 3-arm, phase 2 study of temsirolimus (CCL-779) in combination with letrozole in post-menopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 2007; 23:16s: abstract 564.
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. New Engl J Med 2011;364: 514–23.
- Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis placebo-controlled trial. Lancet 2013;1:817–24.
- Ohtsu A, Ajani JA, Bai YX, Bany YJ, Chung HC, Pan HM, et al. Everolimus for previously treated advanced gastric cancer: Results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935–43.
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer 2010;116:4256–65.
- Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex phase 3 trial. Lancet 2013;1:125–32.
- Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. New Engl J Med 2012;366:520–9.
- Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J Clin Oncol 2012;30:2718–24.
- Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2009;27:2630–7.
- Pavel ME, Hainsworth JD, Baudin E, Peeters M, Horsh D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005–12.
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New Engl J Med 2007; 356:2271–81.
- Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 2013;31:195–202.
- Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: The study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol 2011;65:624–35.
- Ramirez-Fort MK, Case EC, Rosen AC, Cerci FB, Wu S, Lacouture ME, et al. Rash to the mTOR inhibitor everolimus systematic review and meta-analysis. Am J Clin Oncol Epub 2012 Dec 13.
- Gomez-Fernandez C, Garden BC, Wu S, Feldman DR, Lacouture ME. The risk of skin rash and stomatitis with the mammalian target of rapamycin inhibitor temsirolimus: A systematic review of the literature and meta-analysis. Eur J Cancer 2012;48:340–6.
- Hadoux J, Vignot S, De La Motte Rouge T. Renal cell carcinoma: Focus on safety and efficacy of temsirolimus. Clinical medicine insights. Oncology 2010;4:143–54.
- Balagula Y, Rosen A, Tan BH, Busam KJ, Pulitzer MP, Motzer RJ, et al. Clinical and histopathologic characteristics of rash in cancer patients treated with mammalian target of rapamycin inhibitors. Cancer 2012;118:5078–83.
- Gandhi M, Kuzel T, Lacouture M. Eosinophilic rash secondary to temsirolimus. Clin Genitour Cancer 2009;7:E34–6.
- Staehler M, Haseke N, Khoder W, Stief CG. Profile of temsirolimus in the treatment of advanced renal cell carcinoma. Onco Targets Ther 2010;3:191–6.
- Paplomata E, Zelnak A, O’Regan R. Everolimus: Side effect profile and management of toxicities in breast cancer. Breast Cancer Res Treat 2013;140:453–62.
- Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: The Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012;67:1025–39.
