Abstract
Background. Treatment of high-grade osteosarcoma remains a major challenge in orthopedic oncology as no major breakthrough in overall survival has occurred in the past 20 years. Due to the rarity of the disease, comparing the results of a single institution to best standard practice needs the establishment of clinical databases. The aim of this study was to report the cumulative 30-years’ experience of a single institution and to assess the incidence, survival and prognostic factors of high-grade osteosarcoma using a recently validated, hospital-based database, representing all citizens living in western Denmark, the Aarhus Sarcoma Registry.
Material and methods. Between 1979 and 2008, 169 patients were treated at the Sarcoma Centre of Aarhus University Hospital for high-grade osteosarcoma. The incidence was calculated as a WHO age-standardized incidence per million per year. The endpoint was overall survival, analyzed by the Kaplan-Meier method and log-rank. Possible prognostic factors were analyzed by the uni- and multivariate Cox proportional hazard method.
Results. The incidence of high-grade osteosarcoma in western Denmark from 1979 to 2008 was 2.7/million inhabitants/year. The five-year overall survival was 42% (95% CI 34; 49) for the whole cohort of patients with high-grade osteosarcoma and 54% (95% CI 43; 64) for patients with localized disease treated with wide excision and chemotherapy. For patients treated with curative intent, no soft tissue extension, treatment with sufficient surgical margin and standard chemotherapy, as well as a high degree of necrosis after chemotherapy were all independent prognostic factors for overall survival.
Conclusion. The data from this hospital-based, validated database confirms the relevance of the known prognostic factors of high-grade osteosarcoma and emphasizes the importance of adequate surgical margins and chemotherapy.
Osteosarcoma is a relatively rare malignancy of the bone that primarily affects adolescents [Citation1]. Most patients with localized disease will die from metastatic disease if only treated with surgical resection [Citation2]. However, the prognosis of patients with localized osteosarcoma was greatly improved with the introduction of multiagent chemotherapy as part of the multimodality management. The five-year overall survival after the introduction of chemotherapy is approximately 50–65% for patients diagnosed with primary localized osteosarcoma [Citation1,Citation3–6], however, 10–30% of the patients will present with metastatic disease at the time of diagnosis [Citation7,Citation8].
Due to the rarity of the disease, large multicenter clinical trials are needed to draw conclusions regarding new treatments and the importance of prognostic factors. For single institutions to be able to evaluate their own results and test them against best international practice there is a need to gather information of non-selected patients in clinical hospital-based databases, representing a geographically defined area.
In this study, we have investigated all patients treated at a single institution, servicing the population of Western Denmark from 1979 to 2008 with the aims of characterizing patients with high-grade osteosarcoma, reporting treatment outcome and evaluating various prognostic factors for overall survival.
Material and methods
Study population
All 169 patients with high-grade osteosarcoma, who had their primary diagnosis in the period 1979 to 2008 at the Aarhus Sarcoma Centre, were included in this study. High grade was defined as either grade III or grade IV and classified by a specialized pathologist at the center according to the WHO criteria at the time. A total of 19 patients with low grade (parosteal osteosarcoma) or intermediate grade (periosteal osteosarcoma) were excluded.
Data sources
The Sarcoma Centre in Aarhus has, since 1979, been a multidisciplinary center for the treatment of sarcoma. Since 1990 virtually all cases of sarcomas in western Denmark, which covers a recruiting area corresponding to 2.5 million people [Citation9], were referred to the center. Management of all patients was undertaken in the same university hospital by a small team of dedicated sarcoma specialists in radiology, pathology, pediatric and adult oncology, and orthopedic surgery. All patients were recorded in the Aarhus Sarcoma Registry (ASR) [Citation10]. Patients were retrospectively included in the registry before 1993 and prospectively hereafter. The database contains comprehensive clinical data including age, sex, anatomic site of primary tumor, histological type, type of surgery, surgical margin (intralesional/marginal or wide/radical) acquired form pathology reports and classified according to Enneking [Citation11], details about chemotherapy and radiation, recurrence or metastasis, salvage therapy, and at least five years of follow-up after primary treatment. Tumor size, and soft tissue extension. Completeness of the patients in the ASR was ascertained through linkage with the Danish Cancer Registry (DCR) [Citation12]. Data retrieved from the DCR included all patients living in western Denmark at the time of diagnosis with tumor located in the bone based on ICD-10 codes: C40, C41, C47 and C49. These data were reviewed by one pathologist with expertise in sarcoma and patient with non-sarcoma pathology were excluded. The Danish Cancer Registry was founded in 1943, and since 1987 the reporting of new cases of cancer in Denmark to the registry has been compulsory. All Danish citizens have a unique civil personal registration number and free of charge healthcare system which means that all information between registries and databases can be linked on an individual level and each individual can be tracked regarding healthcare services, hospital admission, death, emigration etc. The medical files from both the Department of Orthopaedic Surgery and the Department of Oncology were considered to be the golden standard and were reviewed for all patients registered in the ASR by two independent researchers not involved in treatment using a standardized form. Data that were missing or incorrect in the ASR were added or corrected [Citation10]. Uncertainties were discussed with the rest of the authors who constitute a team of sarcoma experts and a consensus was reached. A pathologist specialized in sarcoma pathology was responsible for reviewing the primary pathology report, however, no revision of pathology was done.
Treatment at the Aarhus Sarcoma Centre
The diagnostic program at the Aarhus Sarcoma Centre included radiological evaluation, clinical examination, as well as tumor biopsy which was taken surgically. Treatment strategy was always discussed at a multidisciplinary conference. Throughout the entire period, the primary treatment for localized osteosarcoma was surgery. In the first 10 years (until 1989) chemotherapy was not standard, and many patients underwent amputation without additional treatment. After 1989 chemotherapy was used systematically together with an increasing trend towards limb salvage surgery. Limb salvage was aimed at if a wide margin and a good functional result could be expected, otherwise an amputation was to be preferred. Patients who met the inclusion criteria of the EORTC 80931 and later the EURAMOS 1 protocols were included and treated according to the guidelines of these protocols. Patients, who did not meet the inclusion criteria of the protocols, received standard treatment of the time or a consensus treatment strategy was decided in the multidisciplinary conference. An attempt at removal of lung metastases was done if the patient presented with operable metastases only to the lungs. Patients with disseminated disease beyond the lungs either at diagnosis or during the follow-up were treated with palliative intent using combination chemotherapy, with or without radiotherapy. All patients were followed for at least five years after surgery.
Statistics
The incidence was calculated using data from StatBank Denmark [Citation9]. Age standardized incidence rate using the WHO standard population was calculated as overall and separate for male and female and for patients under the age of 25 years [Citation13]. The endpoint was overall survival, timed from date of referral until death (from any cause) or patients were censored at date of last follow-up if death has not occurred. Overall survival was analyzed by the Kaplan-Meier method and log-rank test. Possible prognostic factors were analyzed for 135 patients treated with curative intent for localized disease. Prognostic factors included; age at diagnosis (< 40/≥ 40 years), soft tissue extension (yes/no), tumor size (cm), location (axial/extremity), “ideal treatment” defined as surgery with wide margin and chemotherapy (yes/no), and necrosis after chemotherapy treatment (< 90%/≥ 90%). Cut points for the continuous variables were selected based on an a priori review of the literature and number of events. Tumor size was analyzed as a continuous variable. Prognostic factors were analyzed as hazard ratios, using the Cox proportional hazard model. For all analyses a 5% level of significance was used in this study. The software program STATA 12 was used.
Ethics
The study has been approved by the Ethics Committee of Denmark and the Danish Agency of Data Protection.
Results
Incidence of high-grade osteosarcoma in western Denmark
The 169 patients included in this study corresponded to 95% of all high-grade osteosarcoma patients in western Denmark from 1979 to 2008. Only eight more patients (diagnosed from 1979 to 1994) were found in the Danish Cancer Registry but not included in the database. The hospital records of these extra eight patients could not be retrieved. The WHO age-standardized IR in western Denmark in the period 1979–2008 was 2.7/million inhabitants/year (95% CI 2.3; 3.0). The incidence in the age group younger than 25 years was 4.6/million/year, 6.2/million/year for males and 3.1/million/year for females.
Patients and tumor characteristics
The median age was 20 years (range 4–81 years) and 61% were males. summarizes patients and tumor characteristics according to the time of referral. The number of patients admitted to the center was constant all through the study period. The histological diagnosis included 152 patients with conventional osteosarcoma (chondroplastic, fibroblastic, osteoblastic), six patients with telangiectatic osteosarcoma, three patients with small cell osteosarcoma and eight patients with histological type that was not specified. Radiation-induced osteosarcoma comprised 4.7% of the patients. A total of 140 patients had localized disease at the time of diagnosis (83%), while 29 (17%) had distant metastasis. Nineteen of these had only lung metastasis while 10 had distant metastasis to other sites/organs with or without lung metastasis.
Table I. Patients characteristic and treatment modalities of high-grade osteosarcoma in western Denmark in the time period 1979–2008 (n = 169).
Treatment characteristics
Treatment characteristics are shown in . A total of 143 patients (134 localized and 9 metastatic) were treated with radical intent while 26 patients were treated palliatively. From 1979 until 1989, 79% of the patients treated with curative intent underwent amputation, and chemotherapy was only given to 29% of the patients, while in the last decade (1998–2008) limb preservation surgery was used in 77% of the patients, and chemotherapy was administered in 92% of the cases. Of the 143 patients who were treated with radical intent, 88 received an ideal treatment with radical surgical excition achieving wide margin and optimal chemotherapy while 53 patients had either inadequate margin or suboptimal chemotherapy. Nine patients with metastasis at the time of diagnosis were treated with radical intent and seven had radiological complete remission with no evidence of disease after primary treatment. A total of 134 patients (94%) did achieve complete remission after primary treatment, however, 81 patients experienced relapse.
Table II. Treatment modalities of high-grade osteosarcoma in western Denmark in the time period 1979–2008 (n = 169).
Overall survival
The five-year overall survival for the whole cohort of 169 patients was 42% (95% CI 34; 49) as seen in . Patients treated with radical intent and adequate surgical margin but without any chemotherapy had a five-year overall survival of 38% (95% CI 24; 52) compared to a five-year overall of 54% (95% CI 43; 64) when the patients were treated with adequate surgical margin and chemotherapy as shown in [hazard ratio = 0.46 (95% CI 0.30; 0.70), p < 0.001].
Figure 1. Overall survival for all high-grade osteosarcoma patients (n = 169) in the time period 1979–2008.
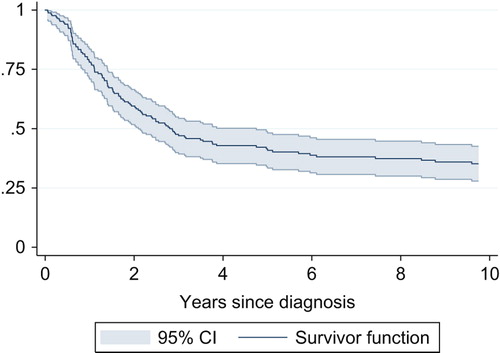
Figure 2. Predicted overall survival for patient treated with curative intent by treatment (A) and year of diagnosis (B), using the univariate Cox proportional hazard regression model (n = 135). Standard treatment was regarded as wide excision combined with optimal chemotherapy. Inadequate treatment included all curative treatment other than standard treatment.
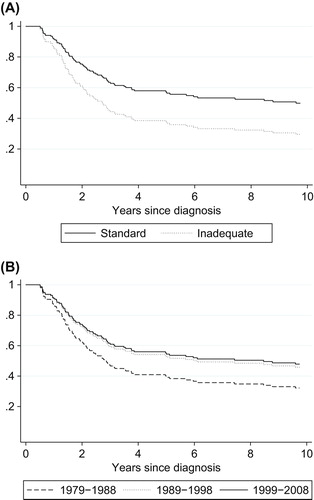
shows the survival curve for patients treated with curative intent who were admitted to the center in different time periods and shows the improved survival after the introduction of routine chemotherapy as standard.
Prognostic factors
show the uni- and multivariate analysis of the various prognostic factors tested in the 135 patients treated radically for localized disease.
Table III. Uni- and multivariate analysis of possible prognostic factors for overall survival (OS) in all patients with high-grade localized osteosarcoma treated with curative intent (n = 135).
These results showed that soft tissue extension, combined chemotherapy and surgery, and degree of necrosis after chemotherapy were independent prognostic factors for overall survival of primary high-grade osteosarcoma.
Patients with soft tissue extension of their primary tumor had a significantly lower overall survival rate compared to patients without soft tissue extension with five-year overall survival of 45% (95% CI 35; 53) and 68% (95% CI 47; 82), respectively.
The degree of necrosis after primary chemotherapy was only reported for 65 patients. Patients with ≥ 90% necrosis after neoadjuvant chemotherapy had a significant better overall survival compared to patients with < 90% necrosis after neoadjuvant chemotherapy.
Relapse
The overall survival rate for patients who relapsed more than one year after primary diagnosis was significantly better than for patients who relapsed within one year with five-year overall survivals of 17.4% (95% CI 9; 28) and 2.6% (95% CI 0.5; 8), respectively.
Discussion
This is a hospital-based single institutional retrospective study of 169 patients with high-grade osteosarcoma, representing 95% of all cases of high-grade osteosarcoma in the western Denmark in the period 1979–2008. The main strength of our study is that the patients were treated in the same institution by a small team of sarcoma specialists, and that the variables evaluated were individually validated for accuracy and were available for almost all patients. The main shortcoming is that the data were collected over a 30-year period during which different drugs, radiological techniques and surgical procedures have been introduced and used.
The age-standardized incidence of osteosarcoma in the western Denmark is 2.7/million inhabitants/year, with a high incidence among males younger then the age of 25 years, which corresponds well to the finding of Whelan et al. [Citation14] and Mirabello et al. [Citation15,Citation16]. In this study the peak incidence was between 10 and 20 years, which corresponds well to the adolescents’ growth spurt and previously published data [Citation1].
The male to female ratio and the most frequently affected locations were in agreement with the available literature reported here is as seen in other studies [Citation4,Citation17]. A change in the therapeutic strategy over the last three decades was seen in the reduction of amputation frequency and the systemic use of chemotherapy; however, an amputation frequency of 33% in the last decade is still higher than expected from literature review. The reason could be certain surgeon and patient preference with regard to expected functionality over cosmetic consideration.
A significantly wider range of effective chemotherapy regimens was available from 1989 and as expected and reported elsewhere this was associated with reduced risk of metastasis compared to patients who had amputation alone. There was no difference in overall survival between amputation and resection when chemotherapy was given.
The five-year overall survival rate of patients with primary localized high-grade osteosarcoma in this study was 54%. This is similar though towards the lower end of what are reported elsewhere [Citation18,Citation21]. As this study found that the overall survival rate was significantly better for patients who adhered to ideal treatment with wide excision and standardized chemotherapy, compared to all the patients treated at the Aarhus Sarcoma Centre. A finding that illustrates the importance of adherence to a clinical practice that resemble protocolized treatment. It has to be mentioned, however, that we did not standardize to age-specific survival.
The relapse rate of 55% and the median time to relapse of 11 months reported in this study are in accordance with the literature [Citation19,Citation20]. The five-year overall survival rate after relapse has been shown to be about 20% [Citation20,Citation21]. In this study we found that the prognosis after first relapse was dependent on the time between date of diagnosis and date of relapse and that the five-year overall survival rate was significantly better for patients with late relapse compared to patients with early relapse (within one year after primary diagnosis), which has been published elsewhere [Citation19,Citation22,Citation23].
In the present study, gender was not significant prognostic variable while the stage of the disease at the time of diagnosis was a prognostic factor. Both finding are in agreement with other studies [Citation17,Citation24–28].
More controversial is that the overall survival for patients under the age of 40 was not significantly better than for patients aged 40 or older. Though this has been shown by other studies [Citation1,Citation28,Citation29] many consider age to be detrimental to outcome because osteosarcoma in older patients is most likely associated with a combination of unusual tumor location, delayed diagnosis, difficulties with surgery, the use of less aggressive chemotherapy, and the presence of comorbidity. Older patients also have more axial bone tumors than younger patients [Citation22,Citation29], which is also the case in our study. The prognostic significance of location in the extremities remains controversial. A large study by Bielack et al. showed a significantly poorer outcome for patients with tumors located at the proximal humerus and proximal femur [Citation28]. Our data did not show a better overall survival rate for patients with tumors located in the extremities compares to axial located tumors.
The relatively small number of patients included in this analysis may be the reason why neither age nor tumor location was found to be independent significant prognostic factors affecting survival. It is to be noted however that patients receiving palliative treatment because of advanced stage and who were excluded from the prognostic factor analysis were older (median age 56 years) and the majority of whom (16 of 26) had axial tumors. Also when looking at the whole cohort (169 patients) both age and location were prognostic factors in the univariate analysis (data not shown).
Tumor size has been suggested as a prognostic factor [Citation5], with different proposed cut points and ways of measuring tumor size [Citation28]. In our study, tumor size was analyzed as a continuous variable, and no significant relation between tumor size and overall survival was seen, which harmonize well with other studies [Citation17,Citation24].
This study showed a clear advantage of adding chemotherapy to the treatment regardless of the type of surgery. The degree of necrosis was also a significant prognostic factor for overall survival. In our study 58% of the patients, for whom the degree of necrosis were measured, had a low response to chemotherapy (< 90% necrosis). In conclusion we found that soft tissue extension at diagnosis, wide excision and standardized chemotherapy, and necrosis > 90% after chemotherapy treatment were independent prognostic factors for overall survival in primary high-grade osteosarcoma. The data from this hospital-based, validated database confirms the validity of the known prognostic factors of high-grade osteosarcoma, emphasized the importance of adequate surgical margin and chemotherapy and described the outcome of localized high-grade osteosarcoma treatment in everyday clinical practice outside clinical trials.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National cancer data base report. Clin Orthop Relat Res 2007;459:40–7.
- Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986;314:1600–6.
- Souhami RL, Craft AW, Van der Eijken JW, Nooij M, Spooner D, Bramwell VH, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet 1997;350:911–7.
- Sampo M, Koivikko M, Taskinen M, Kallio P, Kivioja A, Tarkkanen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland – a nationwide population-based study. Acta Oncol 2011;50:1206–14.
- Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, et al. Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol 2008;97: 456–61.
- Massimo B, Giovanni G, Stefano F, Eleonora B, Adalberto Bdel P, Sandra A, et al. Phase 2 trial of two courses of cyclophosphamide and etoposide for relapsed high-risk osteosarcoma patients. Cancer 2009;115:2980–7.
- Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2003;21:2011–8.
- Petrilli AS, de Camargo B, Filho VO, Bruniera P, Brunetto AL, Jesus-Garcia R, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: Prognostic factors and impact on survival. J Clin Oncol 2006;24: 1161–8.
- Statistic denmark, population denmark [Internet]. 2013. Available from: http://www.statistikbanken.dk [cited 2013 Dec 1].
- Maretty-Nielsen K, Aggerholm-Pedersen N, Keller J, Safwat A, Baerentzen S, Pedersen AB. Population-based aarhus sarcoma registry: Validity, completeness of registration, and incidence of bone and soft tissue sarcomas in western Denmark. Clin Epidemiol 2013;5:45–56.
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980;(153):106–20.
- The Danish Civil Registry, Danish Act on the Civil Registration System [Internet]. 2013. Available from: http://cpr.dk.
- Age standardization of rates: A new WHO standard [Internet]. Available from: http://www.who.int/healthinfo/paper31.pdf.
- Whelan J, McTiernan A, Cooper N, Wong YK, Francis M, Vernon S, et al. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int J Cancer 2012;131: E508–17.
- Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 2009;125:229–34.
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 2009;115:1531–43.
- Schwarz R, Bruland O, Cassoni A, Schomberg P, Bielack S. The role of radiotherapy in oseosarcoma. Cancer Treat Res 2009;152:147–64.
- Kotz R, Dominkus M, Zettl T, Ritschl P, Windhager R, Gadner H, et al. Advances in bone tumour treatment in 30 years with respect to survival and limb salvage. A single institution experience. Int Orthop 2002;26:197–202.
- Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: Clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol 2009;39:514–22.
- Bielack SS, Kempf-Bielack B, Branscheid D, Carrle D, Friedel G, Helmke K, et al. Second and subsequent recurrences of osteosarcoma: Presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol 2009;27:557–65.
- Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the cooperative osteosarcoma study group (COSS). J Clin Oncol 2005;23:559–68.
- Okada K, Hasegawa T, Nishida J, Ogose A, Tajino T, Osanai T, et al. Osteosarcomas after the age of 50: A clinicopathologic study of 64 cases – an experience in northern Japan. Ann Surg Oncol 2004;11:998–1004.
- Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: The experience of a single institution with 44 patients. Cancer 2006;106:2701–6.
- Bispo Junior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo) 2009;64:1177–86.
- Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23:2004–11.
- Bacci G, Picci P, Ferrari S, Sangiorgi L, Zanone A, Brach del Prever A. Primary chemotherapy and delayed surgery for non-metastatic telangiectatic osteosarcoma of the extremities. Results in 28 patients. Eur J Cancer 1994;30A:620–6.
- Bacci G, Ferrari S, Mercuri M, Longhi A, Fabbri N, Galletti S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities in patients aged 41–60 years: Outcome in 34 cases treated with adriamycin, cisplatinum and ifosfamide between 1984 and 1999. Acta Orthop 2007; 78:377–84.
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776–90.
- Jeon DG, Lee SY, Cho WH, Song WS, Park JH. Primary osteosarcoma in patients older than 40 years of age. J Korean Med Sci 2006;21:715–8.
