Abstract
Background. 18F-FDG-PET/CT has been widely used in the staging of malignant lymphomas, and accepted as a tool for response assessment. Among PET parameters, the most frequently studied is maximal standardized uptake value (SUVmax). Metabolic tumor burden (MTB) is a parameter in which both metabolic tumor volume (MTV) and tumor activity are integrated. Here, we analyzed the prognostic value of SUVmax, SUVsum (sum of the SUVmax), whole-body MTV (MTVwb) and MTBwb from baseline and interim PET/CT in patients with diffuse large B-cell lymphoma (DLBCL).
Material and methods. Twenty-nine patients with histologically proven DLBCL were imaged by PET/CT before treatment (Exam I), and one week after the first dose of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) therapy (Exam II). Biopsy specimens were examined by an expert hematopathologist, the Ki-67 proliferation index (PI) was estimated for each biopsy site from the MIB-1 stained sections. The response evaluation was performed after chemotherapy completion (6–8 cycles).
Results. All patients had one or more visualized lymphomatous lesions on 18F-FDG-PET/CT. The SUVmax of the whole-body (BmSUVmax) was higher than the SUVmax at biopsy site (BxSUVmax) (mean: 20.1 vs. 17.3, p < 0.01). The PI correlated with the BxSUVmax (p < 0.05). One week after chemotherapy, SUVmax, SUVsum, MTVwb, and MTBwb decreased significantly (p < 0.01, respectively), SUVsum, MTVwb and MTBwb at Exam II correlated with chemotherapy response at treatment completion (p < 0.05, respectively).
Conclusion. SUVmax is more accurate to detect tumor aggressiveness than biopsy in DLBCL, since BmSUVmax represents the most aggressive tumor of the patient. Interim PET/CT as early as one week after R-CHOP therapy predicts response. Thus, it could be used as a tool for guidance of risk stratification in DLBCL.
Diffuse large B-cell lymphoma (DLBCL) is a biologically heterogeneous subtype of aggressive non-Hodgkin's lymphoma (NHL) that is curable in approximately 60–80% of the patients in the rituximab era [Citation1,Citation2]. Combination chemotherapy with rituximab is currently the standard treatment in patients with DLBCL [Citation1–4], and approximately 30–40% of patients will relapse after first-line treatment [Citation2,Citation5,Citation6]. More aggressive treatments including intensive chemotherapy and autologous stem cell transplantation after the first remission are required to improve survival of the patients who are at high risk of relapse, since only 30–35% of the relapsed or resistant patients can achieve a prolonged progression-free survival with the salvage therapy [Citation7,Citation8]. The prognosis of patients with DLBCL has been estimated before treatment by the International Prognostic Index (IPI) [Citation9]. However, the treatment outcomes of individual patients within the same IPI group can be considerably different [Citation10]. Indeed, a good strategy might be a first-line risk-tailored therapy in poor prognosis patients. Therefore, defining prognostic markers that can accurately classify patients with DLBCL into appropriate risk groups for relapse is highly important for the disease management.
[18F]2-Fluoro-2-deoxyglucose-positron emission tomography/computer tomography (18F-FDG-PET/CT) has been widely used in the staging of malignant lymphomas, and accepted as a tool for response assessment after the end of the treatment [Citation3]. A high FDG-uptake or a hyper-metabolism of glucose is a surrogate marker of aggressive biology in NHL. Evidence has shown that the residual FDG positivity at the end of therapy is predictive for survival [Citation3,Citation11]. However, the role of interim PET/CT, after a few cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) treatment, for predicting survival of patients with DLBCL is still controversial [Citation12–17]. Some studies showed that interim PET/CT was able to predict the survival of patients with DLBCL [Citation12,Citation14,Citation15], but others have drawn different conclusions [Citation13,Citation16,Citation17]. The prognostic value of baseline and early interim PET/CT for post-treatment response has not been studied.
Among PET parameters, the most frequently studied is maximal standardized uptake value (SUVmax), which is a semi-quantitative measure point of 18F-FDG concentration in the tissue. Here, we evaluated the prognostic value of pre-treatment and early interim PET/CT on post-treatment response (complete or partial response) by using various quantitative metabolic parameters including SUVmax, SUVsum (sum of the SUVmax), whole-body metabolic tumor volume (MTVwb), whole-body metabolic tumor burden (MTBwb), and changes of these parameters after one week of R-CHOP treatment in patients with DLBCL.
Material and methods
Patients
Patients were enrolled from our prospective clinical study investigating the potential of PET/CT and magnetic resonance imaging (MRI) for early chemotherapy response evaluation in patients with NHL. The inclusion criteria were: at least 18 years old, histologically proven DLBCL, WHO performance scale (Zubrod score) better than 4. The exclusion criteria were: concomitant previous malignant disease, primary central nervous system lymphoma, pregnancy or lactation, psychosis, diabetes, human immunodeficiency virus infection or acquired immunodeficiency syndrome, or other serious medical conditions that would prevent the imaging examinations. The study was approved by the Ethics Committee of Tampere University Hospital, and all patients gave written informed consent prior to study entry.
All patients underwent anamnestic and physical examination and standard laboratory tests including the measurement of serum markers such as thymidine kinase (TK), beta 2-microglobulin (B2m), lactate dehydrogenase (LD), and C-reactive protein (CRP). In addition, unilateral bone marrow aspiration and trephine biopsy were performed on each patient. Clinical prognostic indexes, such as Ann Arbor stage and IPI, were also evaluated.
At baseline all 29 patients had one or more visualized lymphomatous lesions on 18F-FDG-PET/CT. The baseline characteristics, clinical staging, and serum markers of the patients are illustrated in . PET/CT detected eight patients with FDG-avid bone lesions, whereas only two of them were found bone marrow involvement by bone marrow biopsy. There was no significant difference of the serum markers between the complete remission (CR) and partial remission (PR) response groups.
Table I. Demographic characteristics, clinical staging, FDG-avid bone lesions and serum markers (mean ± SD) of the 29 patients with DLBCL.
The patients received conventional chemotherapy immediately after the baseline examinations. The typical chemotherapy regimen is CHOP [cyclophosphamide, hydroxydaunorubicin (doxorubicin), Oncovin® (vincristine), and prednisone/prednisolone], which was administered in combination with a monoclonal antibody rituximab (R, MabThera®) at an interval of two or three weeks’ schedule (R-CHOP-14 and R-CHOP-21, respectively). Response evaluation was performed after chemotherapy completion (6–8 cycles) using the revised response criteria described by Cheson et al. [Citation3] that incorporate FDG-PET, immunohistochemistry, and flow cytometry assessments. CR is disappearance of all evidence of disease, and PR means more than 50% regression of measurable disease and no new sites.
Analysis of histological specimens and Ki-67 proliferation index
Biopsies were fixed in 30% formyl saline, processed for paraffin-embedding, sectioned at 5 μm thickness, and stained with hematoxylin and eosin. Immunocytochemical staining was performed with antibodies directed against Ki-67 (monoclonal MIB-1 antibody; DAKO, Denmark; 1/300 dilution; 3 minutes high-power microwave pre-treatment); MIB-1 sections were pre-treated by microwave at high power in buffer. Visualization was achieved using an avidin-biotin complex (ABC) kit (DAKO, Denmark) and diaminobenzidine as the chromogen. Biopsy specimens were examined by an expert hematopathologist and classified according to the WHO/Revised European-American Lymphoma classification of lymphoid neoplasm.
The Ki-67 proliferation index (PI) was estimated for each biopsy site from the MIB-1 stained sections. Two adjacent (× 40 objective) digital photomicrographs were taken from the qualitatively assessed representative region of highest cellular packing density by the same expert hematopathologist. The number of nuclei (excluding endothelium and obvious inflammatory cells) were counted and the PI expressed as the percentage of positive nuclei.
Time points of PET/CT examinations
All patients were followed clinically throughout the study, and they were imaged by whole-body PET/CT before treatment initiation (Exam I), one week after the first dose of chemotherapy (Exam II), and again after chemotherapy completion (6–8 cycles) (example images are showing on Supplementary Figure 1, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.927074).
FDG-PET/CT acquisition and image analysis
All patients underwent an integrated PET/CT (Discovery STE 16, GE Healthcare, Milwaukee, WI, USA) examination. The PET/CT imaging covered a volume from the skull base to the upper thigh, and was acquired approximately 60 minutes after intravenous injection of the 18F-FDG tracer (400 MBq) under fasting conditions. The acquisition was in the three-dimensional (3D) mode with a 128 × 128 matrix and 70 cm field of view (FOV), 3 minutes per bed position. The PET images were reconstructed using the 3D VUE Point reconstruction algorithm (GE Healthcare) with two iterations and 28 subsets. The postfilter used was 6.0 mm FWHM. The acquisition parameters of the CT scanner were: tube voltage, 120 kV; tube current automatic exposure control range, 100–440 mA; noise index, 18.5 HU; rotation speed, 35 mm/rot; pitch, 1.75:1. The CT images were reconstructed to slice thicknesses of 1.25 mm and 5.0 mm. The total examination time for PET/CT was approximately 30 minutes.
The FDG-PET/CT images were evaluated visually and quantitatively. The SUVmax, SUVmean, and MTV were measured from each site (tumor or group of tumors) on the fused PET/CT images using the AW Volume Share™ workstation (GE Healthcare) [Citation18]. AW Volume Share™ allows automatic registration and fusion between two volumetric acquisitions, which come from different acquisition modalities. The image fusion provided added value to side- by-side measurement and interpretation. For each PET/CT dataset, the tumor with the most intense 18F-FDG-uptake among all foci was carefully identified as the SUVmax of the whole-body. The SUVmax at biopsy site was measured retrospectively from each biopsy site or adjacent site for partial excision biopsy. The patient was excluded in case the PET/CT was performed after the biopsy and the biopsied tumor was removed for the purpose of excision biopsy. For each tumor or group of tumors, the MTV was estimated in a 3D manner by selecting volume of interest (VOI) on the axial image, and the size of VOI was manually regulated on the corresponding coronal and sagittal images to include the entire active tumor in the VOI, and an isocontour threshold of 42% of the SUVmax was determined between the background and the maximal pixel value. The SUVmax, SUVmean, and MTV in the VOI were computed automatically by the program [Citation18]. MTB was calculated as the product of SUVmean and MTV. MTVwb is the sum of MTV from all tumors in a patient's body, and the MTBwb is the cumulative MTB of all tumors in a patient's body.
The same PET/CT scanner was used for the serial PET/CT examinations. For the follow-up analysis, the tumor with highest activity in any region or organ at Exam II was used for comparison and as indicator of disease status, even though its location differed from the initial tumor with highest activity on Exam I.
Statistical analysis
The statistical analyses were performed using SPSS software. Paired t-test was used to compare the SUVmax of the whole-body and the SUVmax at biopsy site. Non-parametric Wilcoxon signed rank test was used to compare the quantitative metabolic parameters before and one week after chemotherapy initiation. Mann-Whitney U-test was used to compare the quantitative metabolic parameters between CR and PR response groups. The Spearman's correlation coefficient was used to evaluate the correlations between SUVmax, SUVsum, MTVwb, MTBwb, the absolute change and percentage change of these parameters after treatment, Ann Arbor stage, PI category, and chemotherapy response. All tests were two-sided and p-values less than 0.05 were considered significant.
Results
Seventeen male and 12 female patients with DLBCL (mean age 66 years, range from 32 to 86 years) were qualified for the study. Three male patients (mean age 74 years) dropped out of the second PET/CT examination, and all other 26 patients (14 male 12 female, mean age 65 years, range from 32 to 86 years) were followed by serial PET/CT and clinical examinations.
At baseline, the SUVmax of the whole-body (BmSUVmax) was significantly higher than the SUVmax at biopsy site (BxSUVmax) (20.1 ± 8.3 vs. 17.3 ± 7.7, p < 0.01) in the 29 patients with DLBCL.
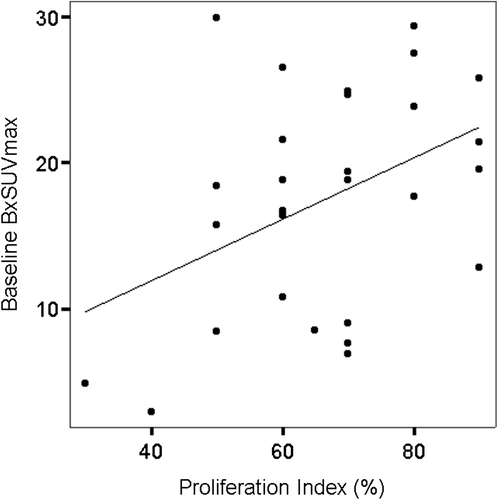
At baseline, the PI correlated with the BxSUVmax (r = 0.42, p < 0.05) (), but not the BmSUVmax (r = 0.28, p = 0.14).
Figure 1. Baseline correlation between proliferation index and SUVmax in 29 patients with DLBCL. Before treatment the proliferation index correlated with SUVmax at biopsy site (BxSUVmax) (r = 0.42, p < 0.05).
Both baseline BmSUVmax (r = 0.45, p < 0.05) and SUVsum (r = 0.46, p < 0.05) correlated with chemotherapy response, and both baseline BmSUVmax and SUVsum in the CR group were lower than those in the PR group (p < 0.05, respectively) ( and Supplementary Figure 2, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.927074). There was no correlation between the Ann Arbor stage, IPI category, and chemotherapy response.
Table II. Quantitative PET/CT parameters before (Exam I) and one week after R-CHOP therapy (Exam II) in 26 patients with DLBCL.
The SUVsum (r = 0.39, p < 0.05), MTVwb (r = 0.42, p < 0.05), and MTBwb (r = 0.40, p < 0.05) at Exam II correlated with chemotherapy response after chemotherapy completion, and both MTVwb and MTBwb at Exam II were smaller in the CR group than those in the PR group (p < 0.05, respectively) (). There was no correlation between BmSUVmax at Exam II and chemotherapy response.
Baseline BmSUVmax correlated with BmSUVmax (r = 0.42, p < 0.05), MTVwb (r = 0.62, p < 0.01), and MTBwb (r = 0.60, p < 0.01) at Exam II. Baseline BmSUVmax also correlated with both ∆BmSUVmax (r = 0.81, p < 0.01) and ∆MTBwb (r = 0.67, p < 0.01) one week after the first R-CHOP therapy. Baseline MTVwb correlated with both ∆MTVwb (r = 0.94, p < 0.01) and ∆MTBwb (r = 0.94, p < 0.01) one week after the first R-CHOP therapy.
One week after chemotherapy initiation, all the evaluated quantitative metabolic parameters including SUVmax, SUVsum, MTVwb, and MTBwb decreased significantly (p < 0.01, respectively) ().
Discussion
18F-FDG-PET/CT provides quantitative information of the tumor metabolic activity, and it is helpful for differentiating viable tumor from post-treatment fibrosis or necrosis. Therefore, it has become an essential imaging tool for management of patients with DLBCL. Previous studies have suggested the association between SUVmax and tumor aggressiveness [Citation19–22], and large lesions and bulky disease have also been reported to be adverse prognostic factors in patients with aggressive NHL [Citation23]. It was recommended that these factors to be routinely considered in risk stratification to decide upon combined therapies. The goal of anti-tumor treatment is to diminish a tumor cell population, ideally to the state of total eradiation. Reduction the number of viable tumor cells can lead to a reduction in anatomical tumor size, and may also be correlated with decreased FDG uptake [Citation24]. The SUVmax reflects the metabolic activity of the most aggressive cell components of the tumor, but it cannot reflect tumor dimensions and volume. However, MTB, i.e. total lesion glycolysis, has been suggested as a quantitative parameter in which both tumor volume and tumor activity are integrated [Citation25]. To identify the optimal markers that could predict chemotherapy response at chemotherapy completion, we compared several quantitative metabolic parameters including SUVmax of the whole-body, SUVsum, MTVwb, and MTBwb from baseline and early interim PET/CT, as indicators that could potentially reflect tumor aggressiveness and overall tumor burden of the whole-body.
Our baseline data showed that SUVmax at biopsy site correlated with proliferation index, which support the concept that SUVmax is associated with tumor aggressiveness in untreated NHL [Citation19–21]. In addition, The SUVmax of the whole-body was significantly higher than the SUVmax at biopsy site in this group of patients with DLBCL. This indicates that PET/CT is more accurate to detect tumor aggressiveness than biopsy, since SUVmax of the whole-body represents the most aggressive tumor of the patient, whereas biopsy site may not be the most aggressive tumor because biopsy is usually performed at site with easy access. The results of this study suggest that the metabolic information obtained by using the SUVmax of the whole-body may help to compensate the limited sampling of histological examination at the biopsy site in patients with lymphomas. In our study, FDG-PET/CT detected eight patients with bone lesions, and bone marrow biopsy found that only two of them had bone marrow involvement. FDG-PET/CT is superior to bone marrow biopsy in detecting bone marrow involvement and leading to upstaging in a proportion of patients with lymphomas [Citation26], since bone marrow biopsy is an invasive diagnostic procedure that allows the analysis of only a very limited area, and bone marrow involvement at locations other than the iliac crest can consequently be missed.
Response to treatment at chemotherapy completion is another important predictor of survival with the advantage of addressing the management for the individual patient [Citation3,Citation11]. Baseline SUVmax of the whole-body had a moderate correlation with chemotherapy response at treatment completion. However, SUVmax of the whole-body lacks prognostic significance one week after chemotherapy because it reflects only the metabolic activity of the most aggressive cells of the tumor, but not the overall tumor activity. However, SUVsum is the sum of the SUVmax of all tumors in a patient's body and represents the overall tumor activity. In our study, SUVsum both at baseline and after the first week of R-CHOP treatment correlated with chemotherapy response. In addition, our study showed that after the first week of R-CHOP treatment MTVwb and MTBwb correlated with chemotherapy response. MTVwb represents the amount of highly metabolic cells of the active tumor, and it has been reported to be an important independent prognostic factor that can complement SUV-based assessments in malignancies [Citation27]. Not only the MTVwb (bulky) is related to the response, but the tumor activity also matters, since MTB accounts for both tumor FDG-uptake and tumor volume.
One week after R-CHOP therapy, all the analyzed quantitative PET/CT parameters decreased significantly in our study, indicating the effectiveness of R-CHOP as a first line treatment in patients with DLBCL. Baseline BmSUVmax also correlated with both ∆BmSUVmax and ∆MTBwb, indicating that the more aggressive the tumor is, the more decrease of the absolute tumor activity and tumor burden after the first week of R-CHOP treatment. Baseline MTVwb correlated with both ∆MTVwb and ∆MTBwb, indicating that the larger the tumor is, the more decrease of tumor volume and tumor burden after the first week of R-CHOP therapy. However, the patients with higher BmSUVmax and MTVwb remained with more aggressive and larger residual tumor/tumors after the first week of R-CHOP treatment, since the baseline BmSUVmax correlated with BmSUVmax, MTVwb, and MTBwb at Exam II.
To find markers that are able to predict an unfavorable response early during R-CHOP treatment is an attractive option and may have important clinical implications. Interim PET/CT was performed after 2–4 cycles of chemotherapy in most previous studies [Citation12,Citation14,Citation15]. If patients undergo interim PET/CT too late, the interim PET/CT will lose its significance for guiding treatment in the early phase. Thus, the interim PET/CT was performed as early as only one week after chemotherapy initiation in our study, and the early interim PET/CT predicted response at chemotherapy completion by yielding a functional indication of lymphoma chemo-sensitivity (identified CR and PR). Thus, very early interim PET/CT could be used as a tool for guidance of risk stratification in DLBCL. The identified quantitative interim PET/CT parameters (SUVsum, MTVwb, and MTBwb) might be novel prognostic markers with clinical implication in risk stratification and in appropriate management of patients with DLBCL. This is a small study with only 29 patients, and the results need to be confirmed in larger studies in the future.
In conclusion, baseline SUVmax indicates tumor aggressiveness, and combined assessment of tumor volume and metabolic activity as early as one week after R-CHOP therapy is predictive of response at chemotherapy completion. These results could serve as a basis for use of very early interim PET/CT in clinical practice, as an adjunct to IPI for tailoring the intensity of treatment to individual patients.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.927074
Download PDF (1.1 MB)Acknowledgements
We would like to thank research nurse Emmi Vettenranta and research coordinator Irja Kolehmainen.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by the Biomedical Image Quantification/University Alliance of Finland and the Elna Savolainen Fund of Tampere University Hospital Research Center.
References
- Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2005;23: 4117–26.
- Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open- label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013–22.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86.
- Sonet A, Bosly A. Rituximab and chemotherapy in diffuse large B-cell lymphoma. Expert Rev Anticancer Ther 2009;9:719–26.
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20 + B-cell lymphomas: A randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105–16.
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010;116:2040–5.
- Moskowitz CH, Schoder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol 2010;28:1896–903.
- Greb A, Bohlius J, Trelle S, Schiefer D, De Souza CA, Gisselbrecht C, et al. High-dose chemotherapy with autologous stem cell support in first-line treatment of aggressive non-Hodgkin lymphoma – results of a comprehensive meta-analysis. Cancer Treat Rev 2007;33:338–46.
- A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987–94.
- Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol 2006;24:995–1007.
- Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol 2011;29:1844–54.
- Yang DH, Min JJ, Song HC, Jeong YY, Chung WK, Bae SY, et al. Prognostic significance of interim 18F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer 2011;47:1312–8.
- Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: Poor predictive value of international harmonization project interpretation. J Nucl Med 2011;52:386–92.
- Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: An early prognostic tool for predicting patient outcome. Blood 2005;106:1376–81.
- Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol 2005;16: 1514–23.
- Yoo C, Lee DH, Kim JE, Jo J, Yoon DH, Sohn BS, et al. Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol 2011;90:797–802.
- Pregno P, Chiappella A, Bello M, Botto B, Ferrero S, Franceschetti S, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 2012;119:2066–73.
- Wu X, Dastidar P, Pertovaara H, Korkola P, Jarvenpaa R, Rossi M, et al. Early treatment response evaluation in patients with diffuse large B-cell lymphoma – a pilot study comparing volumetric MRI and PET/CT. Mol Imaging Biol 2011;13: 785–92.
- Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol 2005; 23:4643–51.
- Watanabe R, Tomita N, Takeuchi K, Sakata S, Tateishi U, Tanaka M, et al. SUVmax in FDG-PET at the biopsy site correlates with the proliferation potential of tumor cells in non-Hodgkin lymphoma. Leuk Lymphoma 2010;51: 279–83.
- Ngeow JY, Quek RH, Ng DC, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol 2009; 20:1543–7.
- Nguyen NC, Kaushik A, Wolverson MK, Osman MM. Is there a common SUV threshold in oncological FDG PET/CT, at least for some common indications?A retrospective study. Acta Oncol 2011;50:670–7.
- Wilder RB, Rodriguez MA, Ha CS, Pro B, Hess MA, Cabanillas F, et al. Bulky disease is an adverse prognostic factor in patients treated with chemotherapy comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy for aggressive lymphoma. Cancer 2001;91:2440–6.
- Fuss M. Strategies of assessing and quantifying radiation treatment metabolic tumor response using F18 FDG positron emission tomography (PET). Acta Oncol 2010;49:948–55.
- Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999;2:159–71.
- Schaefer NG, Strobel K, Taverna C, Hany TF. Bone involvement in patients with lymphoma: The role of FDG-PET/CT. Eur J Nucl Med Mol Imaging 2007;34:60–7.
- Song MK, Chung JS, Shin HJ, Lee SM, Lee SE, Lee HS, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol 2012; 91:697–703.
