Abstract
Background. In radiotherapy (RT) of urinary bladder cancer, the use of intensity-modulated RT (IMRT) opens for sparing of considerable intestinal volumes. The purpose of the present study was to investigate the acute and late toxicities following either conformal RT (CRT) or IMRT for bladder cancer, and to correlate the toxicities to dose-volume parameters.
Material and methods. The study included 116 consecutively treated patients with muscle-invasive bladder cancer who received either CRT (n = 66) or IMRT (n = 50) during 2007–2010. Acute side effects were retrospectively collected whereas late effects were assessed by a cross-sectional evaluation by telephone interview of 44 recurrence-free patients. Acute and late toxicities were scored according to the Common Terminology Criteria for Adverse Event (CTCAE) version 3.0.
Results. Acute diarrhoea grade ≥ 2 was more frequent in patients treated by CRT (56%) compared to IMRT (30%) (p = 0.008). Logistic regression analysis showed a correlation between acute diarrhoea and bowel cavity dose-volume parameters in the 10–50 Gy range. Severe late toxicity (grade ≥ 3) was recorded in 10% of the total cohort, with no statistical difference between the IMRT and CRT groups.
Conclusion. Patients treated with IMRT for bladder cancer had significantly less acute diarrhoea compared to those treated with CRT, but there was no significant difference in late morbidity between the groups. The risk of acute diarrhoea was related to the volume of bowel irradiated.
The aim of radiotherapy (RT) of urinary bladder cancer is to achieve local tumour control with the lowest risk of adverse effects, in particular related to the bowel which represents the primary organ at risk. Introduction of methods to reduce normal tissue irradiation could pave the way for either escalation of the radiation dose or additional systemic therapy, with the aim of improving the therapeutic ratio [Citation1,Citation2].
Aggressive chemo-RT regimens have been introduced for bladder cancer. The primary aim of these regimens is to ensure optimal disease control and at the same time to preserve the bladder. In this sense, the chemo-RT or bladder-conserving strategy are favourable alternatives to radical cystectomy. Bladder conserving chemo-RT strategies comprise extensive transurethral resection, neo- and concomitant chemotherapy and high dose RT to the bladder. Compared to RT alone, these strategies come with an increased risk of acute side effects whereas the risks of late effects are more or less the same [Citation3–5]. Due to the increased risk of acute effects, bladder conserving chemo-RT can only be offered to patients with bladder cancer in good overall health condition who may also be eligible for radical cystectomy. However, even in cohorts of patients in good overall health condition, up to 19% may not receive the full dose of chemotherapy because of acute bowel toxicity [Citation4]. Implementation of bowel sparing strategies, such as intensity-modulated RT (IMRT) as well as image-guided and adaptive strategies may reduce the overall toxicity for bladder cancer patients as well as allow for more intensive chemotherapy as well as for escalation of the RT dose.
In treatment planning studies, IMRT have been shown to reduce the dose to normal tissues compared to CRT for a number of tumour sites including the bladder [Citation2,Citation6–8]. Whether these improvements translate into a reduction in morbidity has not been tested in randomised controlled trails. Some studies suggest that IMRT of pelvic tumours results in a reduction of both acute and late gastrointestinal (GI) morbidity [Citation9–12]. This has in particular been shown in RT for prostate cancer where dose-escalation and improved clinical outcome has become possible due to the improved dose conformity by IMRT [Citation10,Citation13,Citation14].
In a previous treatment planning study of bladder cancer, we demonstrated improved target dose conformity and a significant reduction of the dose to the bowel by IMRT compared to CRT [Citation2]. In the present study we compare normal tissue morbidity rates in bladder cancer patients treated at two different institutions over the same time period, where one institution according to local policy used IMRT and the other used CRT.
Material and methods
IMRT and CRT patient cohorts
Between January 2007 and June 2010, 50 patients with bladder cancer were consecutively treated with IMRT and 66 patients with CRT at Aarhus and Aalborg University Hospitals, respectively. The same diagnostic workup, staging and evaluation of the patients were performed at the urology departments of the two hospitals. This included a CT urography, cystoscopy, blood samples and a transurethral resection of the bladder (TURB) in general anaesthesia to allow thorough transurethral resection and clinical staging of the tumour. All patients included in the study were considered unfit or refused surgery and they had all been discussed at multidisciplinary uro-oncology board meetings. Twenty-one patients (11 from the IMRT group and 10 from the CRT group) refused to undergo cystectomy and received RT. There were no clinical differences in pre- treatment characteristics between the two patient cohorts (). The main clinical characteristics of Transitiocellular carcinoma was found in 113 (97%) patients and 75 (65%) had unifocal bladder tumour. The median age was 75 years (range, 46–85 years) with 84% of the patients being male. The clinical characteristics were overall similar in the two cohorts, with the exception of the presence of carcinoma in situ of the bladder that was more frequent in the CRT group. The lymph nodes were included as a target in 42 (84%) patients in the IMRT group compared to 39 (59%) patients in the CRT group (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.928418).
Table I. Patient characteristics.
RT planning
All patients were treated in supine position using a heel and knee fixation system. A treatment planning CT scan was acquired with intravenous contrast and it was reconstructed with a 3 mm inter-slice distance. In both centres, treatment planning was performed by the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA, USA). The patients were instructed to empty their bladder prior to CT scanning and each treatment fraction.
In the IMRT cohort, the CTV-B included both the bladder (wall and contents) and the tumour. The CTV of the lymph nodes (CTV-LN) included vascular structures in the pelvis with a 5–10 mm margin modified by bone and muscles distal from the promontory in cranial direction to the obturator foramen in the distal direction. All lymph node stations except for the pre-sacral and para-rectal lymph nodes below the sacro-iliacal joint were included. A non-isotropic margin was applied to the CTV-B (28 mm anterior and superior, 20 mm posterior 18 mm inferior, 15 mm lateral) [Citation15] to create the planning target volume of the bladder (PTV-B). A 5 mm margin (8 mm superior and inferior) was added to the CTV-LN to create the PTV-LN. A simultaneous integrated boost IMRT technique was used to deliver the prescribed 60 Gy to the PTV-B and 48 Gy to the PTV-LN over 30 fractions [Citation2].
In the CRT cohort, the clinical target volume of the bladder (CTV-B) included the bladder and the tumour (same definition as above). The treatment field edges of the elective lymph node volume (CTV-LN) was defined anatomically by the pelvic inlet in cranial direction, 1 cm below the obturatory foramen in caudal direction, 1.5 cm lateral of the linea terminalis in lateral direction, 2 cm anterior of the sacrum in dorsal direction and with a margin of at least 2 cm to the bladder in the ventral direction. The treatment consisted of a four-field box technique with fields shaped by multileaf collimators (MLCs). A dose of 60 Gy in 30 fractions was prescribed to the CTV-B with a 2 cm isotropic margin to the MLCs. The dose to the elective lymph node volume was 46 Gy in 23 fractions.
In all patients, the bowel cavity was contoured according to Sanguineti et al. [Citation16] from the upper level of the fifth lumbar spine to the last CT slice that included a bowel segment limited anterior by the abdominal wall and lateral to the pelvic wall, excluding the bladder and rectum. Rectum was delineated from the recto-sigmoid junction in cranial direction and included the anus in distal direction. The specific rectal morbidity and dose-volume characteristics was not analysed in this study. All contouring was reviewed as part of the present study.
Morbidity scoring
Acute toxicities and prescription of anti-diarrhoea medication were retrospectively scored and recorded for all patients, from their treatment charts. As part of the treatment policy, all patients were regularly evaluated for toxicity and overall condition at baseline, midway and at the end of treatment.
Severe CTCAE late grade 3 or more toxicity (typically involving hospitalisation) was scored using individual patient charts from the end of RT to November 2010. Additionally, we gathered a cross-sectional status on late toxicity by telephone from patients alive and without recurrence at the time of follow-up, including 49 patients [42% of the total population; 21 of 50 (42%) in the IMRT cohort and 28 of 66 (42%) in the CRT group]. Five of these patients were not interviewed due to dementia (n = 3), language problems (n = 1) or because they were lost from follow-up (n = 1). Lymph-nodes were included in the target volume in 14 of 19 of these patients (74%) in the IMRT and in 13 of 25 (52%) patients in the CRT group. Median follow-up time was 14 months (range, 4–39 months) for the IMRT patients and 16 months (range, 8–45 months) for CRT patients.
All morbidity was scored according to the Common Terminology Criteria for Adverse Event version 3.0 (CTCAE) [Citation17].
Statistics
The toxicity scores were dichotomised into toxicity grades ≥ 2 (2+) or grade ≥ 3 (3+) and analysed as binominal data presented in percent with 95% confidence interval (CI 95%). Comparisons between groups were analysed by Fisher's exact test for differences. The DVH data are reported as medians and ranges. A non-parametric Wilcoxon rank-sum test was used to test for differences in the volume of bowel cavity receiving more than 10, 20, 30, 40, 50 and 60 Gy (V10Gy − V60Gy) between patients with or without grade 2 + acute diarrhoea. A logistic regression model was used to analyse the effect of irradiation volume as a continuous variable. Finally, estimates of survival for the IMRT and the CRT groups were analysed using the Kaplan-Meier method and compared by use of the log-rank test. In all tests, a significance level of 5% was considered statistically significant. Statistical analyses were performed using the STATA 11 software package (StataCorp. 2009; Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Results
Peak acute toxicity
All patients included in the study completed the RT as planned with abruption of the treatment in only four patients due to morbidity.
In the total patient cohort, acute grade 2 + bowel toxicity mainly consisted of symptoms of diarrhoea (45%; 36–54%), bowel pain (8%; 4–14%), nausea (9%; 4–15%) and proctitis/urgency (2%; 0–6%). Acute grade 2 + urinary toxicities were mainly increased frequency (66%; 57–75%), radiation cystitis (12%; 7–19%), infection (16%; 9–23%) and bladder pain (18%; 12–26%). Acute grade 3 frequency occurred in 20% (13–28%), diarrhoea in 3% (1–9%), bowel pain in 4% (1–10%) and bladder pain in 2% (0–6%). No grade 4 toxicity was observed. The main baseline and acute toxicities in the IMRT and CRT groups are summarised in and Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.928418.
Table II. Baseline and peak acute morbidity.
In the IMRT group, acute grade 2 + diarrhoea occurred in 30% (18–45%) compared to 56% (42–68%) in the CRT group (p = 0.008). The difference was even more pronounced in the patient groups receiving RT of the pelvic lymph nodes (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.928418). No grade 3 diarrhoea was recorded in the IMRT group compared to 6% (2–15%) in the CRT group.
Acute grade 2 + urinary frequency occurred in 66% (51–79%) in the IMRT group and 67% (54–78%) in the CRT group (p = 0.9). Baseline urinary symptoms were frequent in both the IMRT and CRT groups ().
Dose-volume relationship for acute diarrhoea
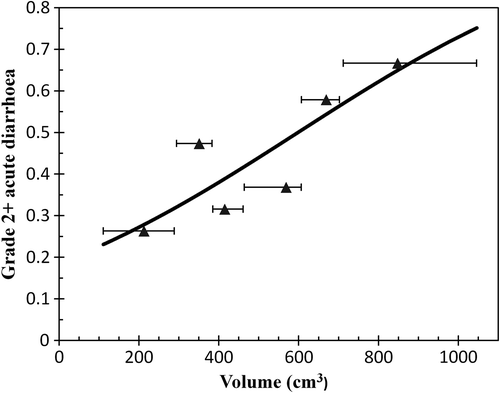
Patients with grade 2 + diarrhoea had significantly larger volumes of bowel irradiated, compared to patients with grade 0–1 diarrhoea, with significant differences between these two groups seen between 10 and 50 Gy (). In a logistic regression, there was a clear relationship between grade 2 + diarrhoea and V45Gy for the bowel cavity (p = 0.007), with a V45Gy of 200 cm3, 400 cm3, 600 cm3 and 800 cm3 corresponding to a risk of grade 2 + diarrhoea of 27%, 38%, 50% and 62%, respectively (). The four patients with grade 3 diarrhoea had a median V45Gy of 667 cm3 (range 259–755 cm3).
Figure 1. Median bowel cavity DVHs for patients with or without grade 2 + acute diarrhoea. Significant difference at 10 Gy, 20 Gy, 30 Gy, 40 Gy and 50 Gy (* p < 0.05).
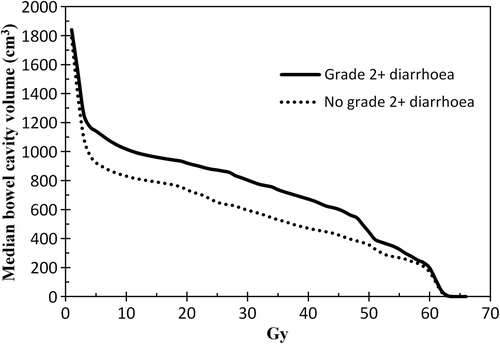
Figure 2. Logistic regression curve for the risk of grade 2 + acute diarrhoea as a function of the bowel cavity receiving a dose of 45 Gy or higher (V45Gy). The individual points represent the risk acute diarrhoea for six groups of 19–21 patients each arranged according increasing volume of bowel cavity receiving a dose of 45 Gy or higher.
Late morbidity and survival
Of the total population, hospitalisation because of severe late grade 3 toxicity (CTCAE) occurred in 12 of the 116 patients (10%), with 4 of 50 (8%) in the IMRT group and 8 of 66 (12%) in the CRT group (p = 0.6). Four patients underwent operation for acute bowel obstruction and one patient had a cystectomy because of intractable bladder symptoms.
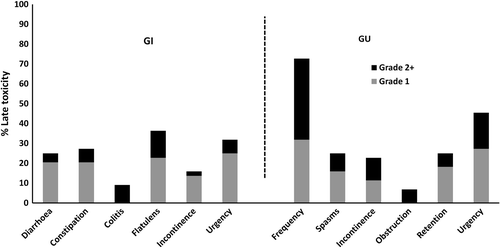
In the cross-sectional study, any late grade 2 + GI toxicity occurred in 17% and 40% of patients receiving IMRT and CRT (p = 0.1), and any late grade 2 + GU toxicity in 63% and 48% of the patients (p = 0.4). Two patients reported grade 3 GI toxicity (one with acute bowel obstruction and one with vascular ischemia of the superior mesenteric artery), both in the CRT group. Eight grade 3 GU toxicities were reported. The incidence of grade 1 and grade 2 + morbidities are shown in . Grade 2 + diarrhoea was reported in 5% and grade 2 + frequency in 41%. No statistical difference in late GU toxicity was observed between the IMRT and CRT groups.
Figure 3. Late gastrointestinal (GI) and genitourinary (GU) toxicity (CTCAE v.3) in the total patient cohort.
Overall survival were comparable for patients treated by IMRT and CRT (Supplementary Figure 1, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.928418). At 12 months, overall survival was 78% for IMRT and 71% for CRT. Dose/volume relationship for acute diarrhoea: Individual DVH and peak acute diarrhoea data are available in Supplementary Table II. (Supplementary Table II, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.928418)
Discussion
The present study on clinical outcome in patients receiving RT for bladder cancer strongly indicates that IMRT reduces grade 2 + diarrhoea by almost 50% compared to CRT. This is in line with observations for other pelvic tumour sites; in an Italian study on whole-pelvis adjuvant or salvage RT after radical prostatectomy, there was also significantly less bowel toxicity after IMRT compared to CRT. The bowel toxicity was even further reduced when RT was delivered with helical tomotherapy [Citation6].
Inclusion of the pelvic lymph nodes as part of the target is controversial in RT for muscle invasive urinary bladder cancer. It has never been proven to be beneficial in terms of survival and inclusion of lymph nodes increases the risk of morbidity [Citation18]. However, surgical studies have revealed a high frequency of lymph node metastasis in muscle invasive bladder cancer and this fact favours inclusion of the pelvic lymph nodes in the irradiated volume [Citation19]. In our study there were more patients receiving lymph node irradiation in the IMRT group compared to the CRT group. The frequency of acute grade 2 + diarrhoea was still lower in the IMRT group (including the lymph nodes) compared to the CRT group (bladder only), but it did not reach statistical significance. A similar observation was presented by Sanguineti et al. who found that IMRT to the prostate and pelvic lymph nodes resulted in a lower late GI toxicity rate compared to CRT of the prostate only [Citation20]. However, these analyses are based on small patient number and a firm conclusion cannot be drawn on this important issue.
In our study, we chose to delineate the whole bowel cavity instead of the individual bowel loops to compensate for day-to-day movements. This method has demonstrated a correlation between volume of irradiated bowel and morbidity which was not found when the analysis was performed based on the delineation of individual bowel loops [Citation16].
In the present study we found that the risk of acute grade 2 + diarrhoea was related to the volume of bowel cavity receiving a radiation dose ranging between 10 Gy and 50 Gy. A bowel cavity V45 Gy of 600 cm3 was found to lead to a 50% risk of grade 2 + diarrhoea, and an extra 100 cm3 (of bowel cavity V45 Gy) resulted in an additional 12% risk for acute diarrhoea. This is consistent with a study by Fiorino et al. [Citation21] where the acute bowel morbidity was analysed in 191 prostate cancer patients receiving whole pelvic RT. They found that acute grade 2 + bowel toxicity was related to the volume of bowel cavity (outside the PTV) receiving a dose from 20 Gy to 50 Gy. A dosimetric analysis of acute GI toxicity by Roeske et al. [Citation22] found that the most important risk factor for development of acute bowel toxicity was the volume of bowel cavity receiving a dose above 45 Gy. Additionally, they reported a 10% risk for acute grade 2 + bowel toxicity with bowel cavity V45Gy above 200 cm3. In our study, a 27% risk of grade 2 + diarrhoea was observed with 200 cm3 of the bowel cavity receiving a dose of 45 Gy or higher. These results underline the importance of reducing the irradiated volume of the bowel cavity as much as possible. A strategy to achieve this goal may be to use IMRT to reduce the volume of normal tissue outside the PTV receiving 45 Gy or more in the cranial part of the pelvis. Furthermore, image-guided and adaptive RT (IGART) may be utilised to reduce the bowel volumes receiving high doses while also compensating for day-to-day changes in volume and shape of the bladder [Citation23–25]. We have recently demonstrated in a planning study that this volume could be reduced by more than 50% by use of advanced IGART [Citation26].
In our study we did not see any difference in the rates of acute GU toxicity between patients treated with CRT versus IMRT. The most obvious explanation for this is that the bladder as being the primary target is not spared by IMRT. The only way to reduce the GU toxicity may be to introduce partial bladder irradiation and even this strategy remains to be proven. A British randomised study did not show that partial RT resulted in less morbidity compared to whole bladder RT [Citation27].
Regarding late toxicities, the crude incidence of late grade 2 + toxicity in the cross-sectional part of this study was generally low and we did not find differences in morbidity between patients treated by IMRT or CRT. However, it should be noted that 25% of the patients had grade 1 + diarrhoea and 73% grade 1 + urinary frequency (). With longer follow-up we may expect to observe increased late GI and GU toxicities. The severe late GU and GI morbidity rates following RT of bladder cancer in other studies vary from 8–36% and 3–6%, respectively, depending on the treatment technique and the scoring system used [Citation18,Citation28,Citation29].
Obvious limitations of the current study are the retrospective nature of the data included with relative short follow-up time and only 44 patients evaluable for late morbidity. However, the treatment groups had well-balanced patient characteristics, work-up and treatment, reflected in the similar survival outcomes. All patients were negatively selected, meaning that radical cystectomy was preferred whenever possible and RT was only offered for patients who were medically unfit for surgery or patients who refused surgery. The number of patients choosing RT as their own preference did not differ between the groups and survival of the patients in the two groups was not statistically different. However, more patients in the CRT group received RT to the bladder only without irradiation of pelvic lymph nodes which may tend to underestimate the toxicity in the CRT arm and which may explain the lack of difference in late toxicity between the groups. The retrospective design and the relatively low number of patients included do not allow firm conclusions based on this analysis, but the study strongly indicated the importance of dose sparing to the intestinal cavity which can be achieved by IMRT.
In conclusion, IMRT for urinary bladder cancer resulted in a significant reduction of acute bowel toxicity (diarrhoea) compared to CRT whereas no difference was found in the crude incidence of late toxicity. The study showed a clear dose-volume effect for acute diarrhoea for the bowel cavity. Approaches to reduce irradiation to the bowel are important in dose escalation and chemo-irradiation strategies.
Supplementary Figure 1
Download Zip (122.5 KB)Acknowledgements
The study was supported by grants from CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology, the Danish Council for Strategic Research, the Danish Graduate School for Clinical Oncology and the Danish Cancer Society. The authors also acknowledge the support from the Department of Medical Physics at both Aalborg and Aarhus University Hospitals.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fokdal L, Høyer M, von der Maase H. Radical radiotherapy for urinary bladder cancer: Treatment outcomes. Expert Rev Anticancer Ther 2006;6:269–79.
- Søndergaard J, Høyer M, Petersen JB, Wright P, Grau C, Muren LP. The normal tissue sparing obtained with simultaneous treatment of pelvic lymph nodes and bladder using intensity-modulated radiotherapy. Acta Oncol 2009; 48:238–44.
- Mitin T, Hunt D, Shipley WU, Kaufman DS, Uzzo R, Wu CL, et al. Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracil-cisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): A randomised multicentre phase 2 trial. Lancet Oncol 2013;14:863–72.
- Müller AC, Diestelhorst A, Kuhnt T, Kuhn R, Fornara P, Scholz HJ, et al. Organ-sparing treatment of advanced bladder cancer: Paclitaxel as a radiosensitizer. Strahlenther Onkol 2007;183:177–83.
- International Collaboration of Trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 Trial. J Clin Oncol 2011;29:2171–7.
- Alongi F, Fiorino C, Cozzarini C, Broggi S, Perna L, Cattaneo GM, et al. IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with post-operative adjuvant or salvage radiotherapy after radical prostatectomy. Radiother Oncol 2009;93:207–12.
- Dogan N, King S, Emami B, Mohideen N, Mirkovic N, Leybovich LB, et al. Assessment of different IMRT boost delivery methods on target coverage and normal-tissue sparing. Int J Radiat Oncol Biol Phys 2003;57:1480–91.
- Griffioen G, Dahele M, Jeulink M, Senan S, Slotman B, Verbakel WF. Bowel-sparing intensity-modulated radiotherapy (IMRT) for palliation of large-volume pelvic bone metastases: Rationale, technique and clinical implementation. Acta Oncol 2012;52:877–80.
- Hasselle MD, Rose BS, Kochanski JD, Nath SK, Bafana R, Yashar CM, et al. Clinical outcomes of intensity-modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2011;80:1436–45.
- Dolezel M, Odrazka K, Vaculikova M, Vanasek J, Sefrova J, Paluska P, et al. Direct comparison of acute and late toxicity with 3D-CRT 74 Gy and IMRT 78 Gy. Strahlenther Onkol 2010;186:197–202.
- Jain S, Loblaw DA, Morton GC, Danjoux C, Szumacher E, Chu W, et al. The effect of radiation technique and bladder filling on the acute toxicity of pelvic radiotherapy for localized high risk prostate cancer. Radiother Oncol 2012;105:193–7.
- Macchia G, Cilla S, Morganti AG, Deodato F, Legge F, Piermattei A, et al. Adjuvant volumetric-modulated arc therapy with simultaneous integrated boost in endometrial cancer. Planning and toxicity comparison. Acta Oncol 2013;53:251–8.
- Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, et al. Long-term results of conformal radiotherapy for prostate cancer: Impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 2008;71:1028–33.
- McDonald AM, Jacob R, Dobelbower MC, Kim RY, Fiveash JB. Efficacy and toxicity of conventionally fractionated pelvic radiation with a hypofractionated simultaneous versus conventionally fractionated sequential boost for patients with high-risk prostate cancer. Acta Oncol 2013;52:1181–8.
- Redpath AT, Muren LP. An optimisation algorithm for determination of treatment margins around moving and deformable targets. Radiother Oncol 2005;77:194–201.
- Sanguineti G, Little M, Endres EJ, Sormani MP, Parker BC. Comparison of three strategies to delineate the bowel for whole pelvis IMRT of prostate cancer. Radiother Oncol 2008;88:95–101.
- National Cancer Institute. Common Terminology Criteria for Adverse Events version 3.0. 9-8-2006. [Internet]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcae_glossary.pdf [Cited 2011 Jan 10].
- Majewski W, Tarnawski R. Acute and late toxicity in radical radiotherapy for bladder cancer. Clin Oncol (R Coll Radiol) 2009;21:598–609.
- Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol 2001;19:666–75.
- Sanguineti G, Cavey ML, Endres EJ, Franzone P, Barra S, Parker BC, et al. Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate-only radiotherapy to 76 Gy?Strahlenther Onkol 2006; 182:543–9.
- Fiorino C, Alongi F, Perna L, Broggi S, Cattaneo GM, Cozzarini C, et al. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys 2009;75:29–35.
- Roeske JC, Bonta D, Mell LK, Lujan AE, Mundt AJ. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol 2003;69:201–7.
- Foroudi F, Wong J, Haworth A, Baille A, McAlpine J, Rolfo A, et al. Offline adaptive radiotherapy for bladder cancer using cone beam computed tomography. J Med Imaging Radiat Oncol 2009;53:226–33.
- Meijer GJ, van der Toorn P-P, Bal M, Schuring D, Weterings J, de Wildt M. High precision bladder cancer irradiation by integrating a library planning procedure of 6 prospectively generated SIB IMRT plans with image guidance using lipiodol markers. Radiother Oncol 2012; 105:174–9.
- Wright P, Redpath AT, Høyer M, Muren LP. A method to individualize adaptive planning target volumes for deformable targets. Phys Med Biol 2009;54:7121–33.
- Vestergaard A, Muren LP, Søndergaard J, Elstrøm UV, Høyer M, Petersen JB. Adaptive plan selection vs. re-optimisation in radiotherapy for bladder cancer: A dose accumulation comparison. Radiother Oncol 2013;109:457–62.
- Huddart RA, Hall E, Hussain SA, Jenkins P, Rawlings C, Tremlett J, et al. Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: Results of the BC2001 trial (CRUK/01/004). Int J Radiat Oncol Biol Phys 2013;87:261–9.
- Tonoli S, Bertoni F, De Stefani A, Vitali E, De Tomasi D, Caraffini B, et al. Radical radiotherapy for bladder cancer: Retrospective analysis of a series of 459 patients treated in an Italian institution. Clin Oncol 2006;18:52–9.
- Hoskin P, Rojas A, Saunders M. Accelerated Radiotherapy, Carbogen, and Nicotinamide (ARCON) in the treatment of advanced bladder cancer: Mature results of a phase II nonrandomized study. Int J Radiat Oncol Biol Phys 2009; 73:1425–31.
