Abstract
Background. Long-term Hodgkin lymphoma (HL) survivors have an increased risk of late cardiac morbidity and secondary lung cancer after chemotherapy and mediastinal radiotherapy. In this prospective study we investigate whether radiotherapy with deep inspiration breath-hold (DIBH) can reduce radiation doses to the lungs, heart, and cardiac structures without compromising the target dose.
Patients and methods. Twenty-two patients (14 female, 8 male), median age 30 years (18–65 years), with supra-diaphragmatic HL were enrolled and had a thoracic PET/CT with DIBH in addition to staging FDG-PET/CT in free breathing (FB) and a planning CT in both FB and DIBH. For each patient an involved-node radiotherapy plan was done for both DIBH and FB, and the doses to the lungs, heart, and female breasts were recorded prospectively. Mean doses to the heart valves and coronary arteries were recorded retrospectively. Patients were treated with the technique yielding the lowest doses to normal structures.
Results. Nineteen patients were treated with DIBH and three with FB. DIBH reduced the mean estimated lung dose by 2.0 Gy (median: 8.5 Gy vs. 7.2 Gy) (p < 0.01) and the mean heart dose by 1.4 Gy (6.0 Gy vs. 3.9 Gy) (p < 0.01) compared to FB. The lung and heart V20Gy were reduced with a median of 5.3% and 6.3%. Mean doses to the female breasts were equal with FB and DIBH.
Conclusion. DIBH can significantly decrease the estimated mean doses to the heart and lungs without lowering the dose to the target in radiotherapy for patients with mediastinal HL.
Hodgkin lymphoma (HL) is the most common malignancy in young adults and mediastinal involvement is common [Citation1]. The prognosis is excellent with cure rates in localised disease of more than 90% [Citation1–3]. Radiation therapy (RT) is very efficient for obtaining permanent local control. Historically, early stage HL was treated with RT alone, and extended field RT, such as mantle field or subtotal nodal irradiation became the standard treatment. However, the extensive RT was clearly associated with substantial long-term side effects, such as cardiac morbidity and secondary cancers [Citation4–10].
The introduction of effective chemotherapy regimens capable of managing microscopic disease and the documentation of serious late effects of the extensive RT led to a reconsideration of the role of RT in the treatment of HL. RT is now a consolidative treatment after chemotherapy, and the aim is to irradiate only the primary macroscopic lymphoma volume. Moreover, the technological advances in imaging and in RT planning and delivery have allowed significant improvements with regard to accurate dose delivery to the target and sparing of critical normal structures. These technologies, including modern imaging, such as positron emission tomography/computerised tomography (PET/CT), three-dimensional conformal radiotherapy (3DCRT), and intensity-modulated radiotherapy (IMRT), make treatment to the initially involved lymph nodes possible, so-called involved node radiotherapy (INRT) [Citation11–13]. Furthermore, radiation doses have been reduced to 20–30 Gy based on well-conducted, large randomised trials [Citation1,Citation2]. The current standard treatment for localised HL is a combination of a short course of chemotherapy and a course of low-dose RT to a limited volume [Citation11,Citation13].
The choice of treatment is based on the balance between the chance of cure and the risk of side effects. Retrospective series indicate that respiratory-adjusted RT of mediastinal HL can reduce the radiation dose to the lungs and the heart in selected patients [Citation14,Citation15].
We initiated a prospective trial to examine the use of respiratory-adjusted image guidance (including staging PET/CT, planning CT and verification imaging) to define the target for RT combined with modern RT delivery. The purpose of the present study is to investigate if deep inspiration breath-hold (DIBH) can reduce the doses to the lungs, heart, female breasts, and cardiac structures without compromising the dose to the target in patients with mediastinal HL.
Material and methods
Patients
Patients diagnosed with HL referred to our institution between March 2010 and November 2012 were eligible for the study. The inclusion criteria were: age ≥ 18, mediastinal RT expected to be a part of the treatment, patient able to comply with the procedures, and a signed consent.
The patients were treated according to the Danish national guidelines dependent on risk factors with 2–6 courses of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), followed by INRT to 20 Gy in 10 fractions or 30 Gy in 17 fractions, according to the results of the GHSG HD10 and HD11 trials [Citation1,Citation2]. Two patients were also included in the “H10 EORTC/GELA/IIL Randomized Intergroup Trial on early FDG-PET scan guided treatment adaptation versus standard combined modality treatment in patients with supradiaphragmatic stage I/II Hodgkin's lymphoma”.
Procedures
Prior to the study, all patients were introduced to the breath-hold procedure during a 30 minute coaching session. During PET/CT scanning, planning CT, and treatment, the patients were guided to achieve a reproducible inspiration level using visual guidance (video goggles or screen), using the RPM® from Varian Medical Systems (Palo Alto, CA, USA), as described elsewhere [Citation16,Citation17]. In summary, the system consists of a small plastic box with reflective markers tracked by an infrared camera. The box is placed on the patient's chest and the inspiration level is then determined by the anterior-posterior displacement of the marker box.
Imaging and contouring
Before the start of chemotherapy, all patients underwent a whole body PET/CT with injection of 400 MBq of 18F-fluoro-deoxyglucose (FDG) one hour before scanning, according to departmental protocol. In addition to the standard whole-body examination in free breathing (FB), a limited PET/CT scan over the mediastinal region was acquired in DIBH while the patient held his/her breath for a total of one minute (acquired as three repeated breath-holds of each 20 seconds). The data were then fused to one PET scan which afterwards was fused with the CT of thorax, also in deep inspiration. Involved PET positive nodes were contoured independently on both FB and DIBH scans.
After completion of chemotherapy, all patients had a RT planning CT in both FB and DIBH. The pre-chemotherapy gross tumour volume (GTV), including the entire nodes with PET avidity, was contoured. This GTV was transferred to the post-chemotherapy planning CT and the clinical target volume (CTV) was contoured, defined as the GTV adapted to the post-chemotherapy anatomy as described previously [Citation11,Citation13]. This procedure was done for both FB scans and DIBH scans, allowing optimal image fusion with the most precise delineation possible of the CTV. The CTV to planning target volume (PTV) margin was 1.5 cm cranio-caudally and 1 cm in the other directions for FB plans and 1 cm in all directions for DIBH plans. The lungs, heart and female breasts were contoured prospectively. The coronary arteries [left anterior descending (LAD), left main (LMA), left circumflex (LC), and right coronary artery (RCA)] as well as the aortic, pulmonic, mitral, and tricuspid heart valves were contoured retrospectively according to published guidelines [Citation18].
Radiation treatment planning and delivery
For each patient, two RT plans were generated, one in DIBH and one in FB, using the fused images from pre-and post-chemotherapy DIBH and FB scans, respectively (). All plans were created with either parallel opposing fields (POP, gantry angle of 0 and 180 degrees) or IMRT in Eclipse (version 10, Varian Medical Systems) with the AAA algorithm using 6 MV photon beams. For POP, 18 MV supplementary fields were occasionally used in order to reduce hot spots. After evaluating the DIBH-RT and FB-RT plan, the plan determined to be the best in terms of target coverage (i.e. PTV) and sparing organs at risk (heart, lungs and female breast) was chosen for the RT delivery. PTV coverage, defined as% of PTV receiving at least 95% dose level (V95%), mean dose to the lungs, heart, female breasts, coronary arteries, and heart valves were calculated for each patient in both DIBH and FB, as well as lung and heart volume receiving ≥ 30 Gy (V30) or ≥ 20 Gy (V20). The plan determined to be the best in terms of target coverage (i.e. PTV) and sparing organs at risk was chosen for the RT delivery.
Position verification was performed before each treatment using x-ray imaging, either daily or for three first days and thereafter weekly, with subsequent adjusting of the set up if systematic errors in positioning were seen [Citation19]. If the treatment was delivered in DIBH, position verification was also performed at DIBH in order to verify the level of inspiration.
Statistical analyses
This protocol was designed as an exploratory study with the primary endpoint being the reduction in mean lung and heart dose. Although no formal power calculation was performed prior to the initiation of the protocol, four patients were planned in DIBH as a pilot study which indicated that the mean heart dose could be reduced from 11.6 Gy in FB to 8.7 Gy in DIBH. For practical considerations, we included as many patients as possible within a two-year period. Based on the pilot study, the current 22 patients would give the study a power of over 0.9 (std = 4 Gy, alpha = 0.05, two-tailed). Wilcoxon signed rank test for non-parametric paired data was used to compare differences for the dependent variables in the DIBH and FB RT plans with a two-tailed significance level of 0.05. Mann-Whitney U-test for non-parametric independent samples was used to retrospectively analyse the doses to the lungs and heart in patients with involvement of the whole mediastinum (WM), defined as CTV extending 3 cm or more below carina in FB, and compared to patients with only upper mediastinal disease (UM), defined as no CTV 3 cm or more below carina in FB. All statistical analyses were performed with the SPSS statistical software v. 19.
Ethical considerations
The study was approved by the regional ethics committee for Copenhagen H-D-2007-0069.
Results
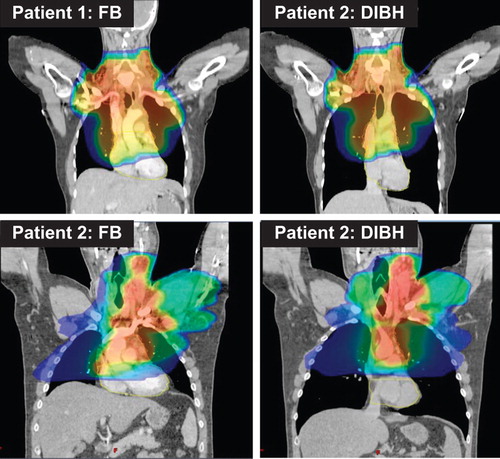
Forty patients were eligible for the study. Of these a total of 22 patients completed all procedures while 13 patients were excluded due to stage III/IV disease or no mediastinal disease, two patients due to technical errors and three patients due to various reasons (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.932435). The characteristics of the participating 22 patients are shown in . Twenty-one of 22 patients had extension of disease below the carina, and 14 of 22 patients had disease 3 cm or more below carina. The FB RT plan was chosen for three patients, and 19 patients had treatment in DIBH. Ten patients were treated using POP and 12 patients using IMRT. illustrates the dose distribution for two patients treated with POP and IMRT, respectively.
Table I. Patient baseline and treatment characteristics.
The DIBH PET/CT scan was done in four breath-holds and the planning CT in one. Patients treated with IMRT had treatment in one breath-hold per field. Patients treated with POP had treatment in one to two breath-holds per field.
The resulting dose estimates with FB and DIBH, respectively, are presented in . There was no statistically significant difference in neither the sizes of CTV or PTV, nor in estimated PTV coverage (V95%), between FB and DIBH plans. However, a trend was seen towards a smaller PTV with DIBH. A statistically significant reduction of the estimated mean doses to the lungs, heart, heart valves and coronary arteries except the RCA was seen (). The reduction in mean dose to the more cranial heart valves, i.e. the pulmonic and aortic valves, was about 20% and the reduction to the more caudal heart valves, the mitral and tricuspid valves, was about 30%.
Table II. Dose characteristics with free breathing (FB) and deep inspiration breath-hold (DIBH).
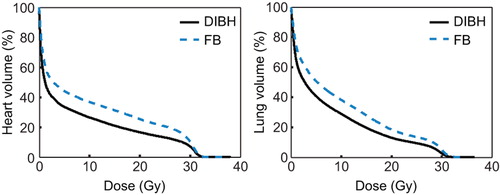
The differences between FB and DIBH mean doses and V20Gy to the heart were most pronounced in the WM group (p < 0.01), cf. . For the whole group the median mean doses to the lungs were 8.5 and 7.2 Gy and to the heart 6.0 and 3.9 Gy with FB and DIBH, respectively. For the WM group, the corresponding values were 15 and 10 Gy to the lungs and 14 and 9.2 Gy to the heart. For the UM group the corresponding values were 3.7 and 2.6 to the lungs and 1.4 and 0.53 Gy to the heart. The reduction in dose with DIBH was observed at all dose levels for the heart and lung suggesting a true dose reduction as opposed to redistribution (see ).
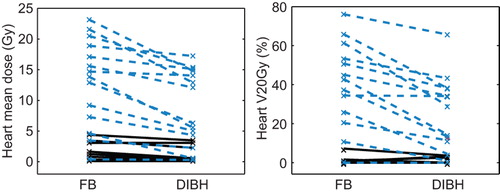
The estimated mean doses to the breasts were not statistically different with FB and DIBH ().
Discussion
DIBH is a simple and well-tolerated approach, in a relatively young and fit patient group, such as patients with HL.
In this prospective study we demonstrate a significant reduction of the estimated radiation doses to the lungs, heart, heart valves, and coronary arteries with DIBH compared to FB. This is in concordance with a previous study, which did not, however, include pre-chemotherapy DIBH imaging [Citation14]. Moreover, we show a more pronounced reduction with DIBH in patients with mediastinal involvement ≥ 3 cm below the carina than in patients with involvement of the upper mediastinum only.
We also demonstrate that IMRT and DIBH can be combined, as was the case for 12 patients in our cohort. Combined IMRT and DIBH results in only marginally longer treatment times compared to FB IMRT, as most fields can be delivered during a single breath-hold. Hence, the treatment appointment time is not increased.
In planning studies it is very important to investigate whether the reduced dose to the lungs and heart in breath-hold is achieved at the expense of an increased dose to other organs. However, with DIBH, there is no spatial redistribution of dose, as opposed to with IMRT, and we found no increase in mean dose to the female breasts. Surprisingly, for some patients the estimated dose to the heart valves is higher with DIBH; however, in the present study the doses to these structures were retrospectively investigated and therefore not considered during the actual RT planning. Thus, incorporation of these cardiac structures in the planning procedure is important if the doses to these organs are to be reduced.
There are several possible mechanisms by which the dose reduction is achieved with DIBH. The use of the pre-chemo PET/CT with DIBH could potentially lead to definition of a smaller target volume and indeed the PET signal seems less blurred in DIBH than in FB in some patients. Although there was a trend towards a smaller CTV volume in DIBH, this difference did not reach statistical significance indicating that this effect has only a minor impact. The increased lung volume, from a median of 2.9 l with FB to a median of 4.9 l, with DIBH means that a substantially smaller proportion of the lungs is included in the PTV with DIBH (see ). All our patients complied very well with the breath-hold procedure and had a very stable and reproducible position during treatment. We therefore reduced the CTV to PTV margin from 1.5 cm to 1.0 cm in the cranio-caudal direction. This will of course reduce the treated normal tissue volume. Finally the heart was pulled caudally with deep inspiration and the mediastinum appeared elongated and narrower on DIBH images (see ). The changes in the anatomy result in a better separation between the heart and the CTV in some patients. All these factors result in a more favourable anatomy for treatment planning for the majority of our patients and especially for patients with more extensive disease in the mediastinum.
Transferring contours from a pre-chemotherapy PET/CT to a post-chemotherapy planning CT is a complex process due to the differences in patient positioning and anatomy (caused by both weight loss and tumour shrinkage) between these two sets of images. With DIBH another level of complexity is added. The present study demonstrates that integration of breathing adapted image guidance into modern staging procedures with PET/CT scanning is feasible. In our study this is made possible because we strictly fuse FB with FB and DIBH with DIBH only. However, in many institutions a pre-chemotherapy DIBH scan will not be available and in these situations extra margins should be added to account for fusion uncertainties [Citation19]. In the future, non-linear fusion tools may be useful if no pre- chemotherapy DIBH scan is available.
Some organs, such as the heart valves, are difficult to contour and some contouring uncertainty may be present, adding some noise to our data. We carefully followed published guidelines [Citation18] in order to reduce this uncertainty and the fact that we investigate differences in the same patient also reduces the effect of contouring uncertainty.
A limitation of this study is that we demonstrate reductions in dosimetric parameters and not in clinical effects. However, considering the fact that the clinical effects of interest have a significant latency of up to several decades, the use of dosimetric surrogates is necessary and justified. We have ample data on late effects due to RT with large fields in patients with HL and relatively reliable estimates of the benefit of a reduced dose to the organs at risk [Citation20]. Any reduction of doses to the organs at risk should be considered beneficial, since we have evidence that no lower dose threshold exists for increased cancer risk, and that the risk of a secondary cancer is dose- volume dependent [Citation8,Citation21–24]. The risk of cardiac disease after radiation is also dose dependent [Citation7,Citation20,Citation24,Citation25], and in one study in breast cancer, the rate of major coronary events increases by 7.4% for each increase of 1 Gy in the mean radiation dose delivered to the heart [Citation25]. Hence, the significant reductions in estimated delivered doses with DIBH compared to FB are likely to be clinically relevant. Clinical testing of the DIBH strategy to exclude inferior tumour control would require a very large phase 3 study, and with an equal PTV coverage we believe that such a trial would be unethical.
The DIBH procedure is simple, most patients can comply with the DIBH procedure, and the PTV coverage can be kept equal. Hence, we recommend that the DIBH technique be offered to patients requiring RT for mediastinal HL. As the DIBH plan may not always be superior to FB, we further recommend that both an FB and a DIBH scan be acquired at treatment simulation in order to compare both treatment strategies.
In conclusion, RT with DIBH reduces the radiation doses to the lungs, heart, and cardiac structures without compromising the dose to the target in a cohort of patients with mediastinal HL. Incorporation of the DIBH procedures in modern imaging and treatment procedures is feasible, allowing optimal imaging for highly conformal RT of mediastinal lymphoma.
Supplementary material available online
Supplementary Figure 1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.932435
ionc_a_932435_sm7918.pdf
Download PDF (273.8 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Eich HT, Diehl V, Gorgen H, Pabst T, Markova J, Debus J, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: Final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 2010;28:4199–206.
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med 2010; 363:640–52.
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage Hodgkin's lymphoma: Final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol 2011; 29:4234–42.
- Ng AK, Bernardo MP, Weller E, Backstrand KH, Silver B, Marcus KC, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol 2002;20:2101–8.
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, van't Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 2003;21:3431–9.
- Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, et al. Second malignant neoplasms among long-term survivors of Hodgkin's disease: A population-based evaluation over 25 years. J Clin Oncol 2002;20:3484–94.
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 1993;270:1949–55.
- Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst 2002;94:182–92.
- Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 2003;290:465–75.
- Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 2003;290:2831–7.
- Girinsky T, van der Maazen R, Specht L, Aleman B, Poortmans P, Lievens Y, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: Concepts and guidelines. Radiother Oncol 2006;79:270–7.
- Girinsky T, Specht L, Ghalibafian M, Edeline V, Bonniaud G, van der Maazen R, et al. The conundrum of Hodgkin lymphoma nodes: To be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol 2008;88:202–10.
- Specht L, Yahalom J, Illidge T, Berthelsen AK, Constine LS, Eich HT, et al. Modern radiation therapy for hodgkin lymphoma: Field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys Epub 2013 Jun 18. pii: S0360-3016(13)00534-8. doi: 10.1016/j.ijrobp.2013.05.005.
- Paumier A, Ghalibafian M, Gilmore J, Beaudre A, Blanchard P, el Nemr M, et al. Dosimetric benefits of intensity-modulated radiotherapy combined with the deep-inspiration breath-hold technique in patients with mediastinal Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys 2012; 82:1522–7.
- Paumier A, Ghalibafian M, Beaudre A, Ferreira I, Pichenot C, Messai T, et al. Involved-node radiotherapy and modern radiation treatment techniques in patients with Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2011;80:199–205.
- Korreman SS, Pedersen AN, Nottrup TJ, Specht L, Nystrom H. Breathing adapted radiotherapy for breast cancer: Comparison of free breathing gating with the breath-hold technique. Radiother Oncol 2005;76:311–8.
- Damkjaer SM, Aznar MC, Pedersen AN, Vogelius IR, Bangsgaard JP, Josipovic M. Reduced lung dose and improved inspiration level reproducibility in visually guided DIBH compared to audio coached EIG radiotherapy for breast cancer patients. Acta Oncol 2013;52:1458–63.
- Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:10–8.
- de Boer HC, Heijmen BJ. eNAL: An extension of the NAL setup correction protocol for effective use of weekly follow-up measurements. Int J Radiat Oncol Biol Phys 2007;67:1586–95.
- Maraldo MV, Brodin NP, Aznar MC, Vogelius IR, Munck af Rosenschöld P, Petersen PM, et al. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal Hodgkin lymphoma. Ann Oncol 2013;24:2113–8.
- Gilbert ES, Stovall M, Gospodarowicz M, van Leeuwen FE, Andersson M, Glimelius B, et al. Lung cancer after treatment for Hodgkin's disease: Focus on radiation effects. Radiat Res 2003;159:161–73.
- Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol 2007;25:1489–97.
- Swerdlow AJ, Schoemaker MJ, Allerton R, Horwich A, Barber JA, Cunningham D, et al. Lung cancer after Hodgkin's disease: A nested case-control study of the relation to treatment. J Clin Oncol 2001;19:1610–8.
- van Leeuwen FE, Klokman WJ, Stovall M, Dahler EC, van't Veer MB, Noordijk EM, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst 2003;95: 971–80.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom- Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98.
