Abstract
Background. Evidence has suggested that radiation therapy with a lower dose per fraction may be a reasonable option for the treatment of centrally located non-small cell lung cancer (NSCLC). The aim of this study was to evaluate the safety and efficacy of two proton beam therapy (PBT) protocols for stage I NSCLC and to determine prognostic factors.
Material and methods. This study included patients clinically diagnosed with stage I NSCLC. Based on the location of the tumor, one of the two PBT protocols was administered. Patients with peripherally located tumors were given 66 Gy relative biological dose effectiveness (RBE) over 10 fractions (Protocol A) while patients with centrally located tumors were given 80 Gy (RBE) over 25 fractions (Protocol B).
Results. Between January 2009 and May 2012, 56 eligible patients were enrolled (protocol A: 32 patients; protocol B: 24 patients). The three-year overall survival (OS), progression-free survival (PFS), and local control (LC) rates were 81.3% [95% confidence interval (CI) 75.9–86.7%], 73.4% (95% CI 67.2–79.6%), and 96.0% (95% CI 93.2–98.8%), respectively. There were no significant differences in outcomes between the two protocols. Late grade 2 and 3 pulmonary toxicities were observed in nine patients (13.4%) and one patient (1.5%), respectively; no grade 4 or 5 toxicities were observed. Sex, age, performance status, T-stage, operability, and tumor pathology were not associated with OS and PFS. Only maximum standardized uptake value (SUVmax; < 5 vs. ≥ 5) was identified as a significant prognostic factor for OS and PFS.
Conclusion. Both high-dose PBT protocols achieved high LC rates with tolerable toxicities in stage I NSCLC patients, and SUVmax was a significant prognostic factor.
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death, with both the highest incidence and mortality rates. While the standard therapy for stage I NSCLC is surgical resection, more than 20% of patients with stage I disease undergo non-surgical treatments. This is because of medical inoperability due to advanced age, comorbidities, or patient refusal. Stereotactic body radiotherapy (SBRT) is currently used to treat patients with early stage NSCLC as it can achieve improved local disease control. However, SBRT is not an option for all patients with early stage NSCLC, including those with centrally located tumors or poor lung function [Citation1–3]. Register et al. compared SBRT to proton beam therapy (PBT) with either passive scattering protons or intensity-modulated protons [Citation4]. Compared with SBRT, they found that PBT significantly reduced the dose to normal tissues for centrally or superiorly located stage I NSCLC. Several reports have also demonstrated that PBT achieves excellent results and minimal toxicities because of its physical properties; however, various doses and fractions were used [total dose range of 51–87.5 Gy; relative biological dose effectiveness (RBE) and dose per fraction of 1.8–16 Gy] [Citation5–14]. Therefore, the optimal PBT dose and fraction number were unclear. In a Japanese multi-institutional review, Onishi et al. reported on 245 patients with stage I NSCLC treated with SBRT [Citation15]. The results showed that local control (LC) and survival rates were better with a biological effective dose of 10 (BED 10) ≥ 100 Gy than with < 100 Gy. Some researchers have suggested that adopting a lower dose per fraction may be reasonable for treatment of centrally located tumors in order to reduce severe toxicities [Citation16,Citation17]. Considering the published findings from various groups, we aimed to perform a comparative analysis of two protocols that differed in dosage and period of treatment. In this study, we investigated the clinical outcomes, toxicities, and prognostic factors related to two high-dose PBT protocols [BED 10 ≥ 100 Gy] for treatment of stage I NSCLC.
Material and methods
Patients
Patients treated in Southern Tohoku Proton Therapy Center were enrolled in this study. Patients who were clinically diagnosed with stage T1 or T2a (≤ 5 cm), N0, M0 NSCLC, according to the 7th International Union Against Cancer TNM classification, were eligible. All patients underwent computed tomography (CT)/positron emission tomography (PET) with 2-(fluorine-18)-fluoro-2-deoxy-d-glucose (FDG-PET/CT) within six weeks before PBT. Histological diagnosis of NSCLC was determined by biopsy with bronchoscopy. If the pathological diagnosis could not be confirmed by biopsy, cases with positive tumor findings by FDG-PET and an increase in tumor size during the observation period were diagnosed with stage I NSCLC by the tumor board, which included diagnostic radiologists, chest surgeons, and pulmonologists; these patients were considered eligible for the study. Tumor operability was also determined by the tumor board. Other eligibility criteria included: age ≥ 18 years; Eastern Cooperative Oncology Group performance status of 0–2; medically inoperable disease or patient refusal of surgery; no previous radiotherapy inside the field of present PBT. Patients with a tumor located close to the stomach or large and small bowels were excluded. The initial workup generally included a medical history and physical examination, laboratory studies (complete blood cell count and comprehensive metabolic panel, blood gas), respiratory function by spirometry analysis, chest radiography, and electrocardiogram. The maximum standardized uptake value (SUVmax) of FDG-PET/CT was obtained by review of the formally dictated radiology report. Informed consent was obtained from all patients. This study was approved by the institutional committee of Southern Tohoku Proton Therapy Center, and the research was in compliance with the Helsinki Declaration.
From January 2009 to May 2012, 56 patients with newly diagnosed clinical stage I NSCLC were treated with PBT at our institution. The median age of patients was 77 years (range 61–89 years). Patients and treatment characteristics are shown in . Protocol A was used in 32 patients (57.1%), and protocol B was used in 24 patients (42.9%). Forty-six tumors [82.2%; 36 adenocarcinoma (64.3%), 10 squamous cell carcinoma (SCC; 17.9%)] were historically diagnosed, and 10 tumors (17.9%) were of unknown histology. Five patients (8.9%) previously underwent surgical resection for lung cancer: one pneumonectomy (1.8%) and four lobectomies (7.1%). Forty-three patients (76.8%) were medically inoperable for the following reasons: 19 for chronic obstructive pulmonary disease (33.9%), eight for high age (14.3%), five for cardiac dysfunction (8.9%), three for interstitial pneumonia (5.4%), three for former pulmonary operation (5.4%), two for brain disease (3.6%), two for renal failure (3.6%), and one for idiopathic thrombocytopenic purpura (1.8%).
Table I. Patient characteristics.
Treatment planning and protocols
All patients underwent simulation at our institution using a 16-slice large-bore helical CT scanner (Aquilion LB; Toshiba, Tokyo, Japan) and a respiratory gating system (Anzai Medical, Tokyo, Japan). Using this system, CT images were obtained in the exhalation phase with a conventional scan at 2-mm slice thickness. A custom-induced vacuum-lock bag (Esform; Engineering System Co., Matsumoto, Japan) was used for patient immobilization. For PBT planning, a 3D treatment planning system (Xio-M; CMS Japan, Tokyo, Japan; Mitsubishi Electric Corporation, Kobe, Japan) was used. The gross tumor volume (GTV) was identified from these images of the pulmonary window setting. The clinical target volume (CTV) included a 5-mm radial expansion of GTV to target possible microscopic disease extension. The CTV was expanded by 5 mm in all directions for setup uncertainty, and by an additional 2–5 mm to compensate for respiratory movements to create the planning target volume (PTV). The total dose at the isocenter was prescribed to cover 90% of PTV. Doses were calculated on the basis of the pencil beam algorithm. Proton energy levels of 150 and 210 MeV for 2–3 portals were planned. The PBT system at the institute (Proton Therapy System; Mitsubishi) used synchrotron, and scattering methods included a bar ridge filter, range shifter, and customized compensator. A respiratory gating system was used to synchronize treatment in the expiratory phase. The RBE of the proton beam was determined to be 1.1.
Dose prescription was chosen after PBT planning. Two protocols were used: protocol A was 66 Gy (RBE) in 10 fractions over two weeks and protocol B was 80 Gy (RBE) in 25 fractions over five weeks. The protocol administered was dependent on the tumor location. Protocol A, prescribed from two or three portals in one day, was the default radiation protocol. Protocol B was used for centrally located tumors, defined as tumors located within 2 cm of the proximal bronchial tree (trachea, carina, main bronchi, and lobar bronchus), major vessels, heart, and esophagus. The daily 3.2 Gy (RBE) was administered from one port in one day. Dose volume constraints for critical structures were as follows: normal lung, the volume exposed to 20 Gy (RBE) or more (V20) < 35% in any dose per fraction; proximal bronchial tree, 80 Gy (RBE) in 3.2 Gy per fraction < 10 cm3; heart, 64 Gy (RBE) in 3.2 Gy per fraction < 10%; esophagus, 64 Gy (RBE) in 3.2 Gy per fraction < 10 cm3; liver, 30 Gy (RBE) < 30% in any dose per fraction. The beam line avoided the spinal cord and brachial plexus. The BED was calculated using a linear-quadratic model with the following formula: BED = nd [1 + d/(α/β)], where n was the number of fractions, d was the dose/fraction, and α/β ratio was 10 Gy.
Follow-up and toxicity evaluation
Chest imaging studies (CT or FDG-PET/CT) and lab analyses were performed every three months after PBT for the first two years and every six months thereafter. When the patients had any pulmonary symptoms, additional radiological analyses were performed to evaluate local failure and radiation pneumonitis. LC was defined as no sign of regrowth or new tumor development in the target area. An increase in FDG uptake was considered a potential sign of local failure. Acute and late toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis and plan evaluations
Baseline characteristics of the patients were compared between the two protocols using the Mann-Whitney rank-sum test for continuous variables and Fisher's exact test for categorical variables. Observations for endpoints began on the date of PBT initiation. Overall survival (OS), progression-free survival (PFS), and LC curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Sex, age (< 70 vs. ≥ 70 years), performance status (0 vs. 2 or 1), T-stage (T1a or T1b vs. T2a), operability, and pathology (adenocarcinoma vs. SCC vs. unknown), and SUVmax (SUVmax < 5 vs. SUVmax ≥ 5) were analyzed for their impact on OS and PFS. All p-values were two-sided, and values of p < 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS software version 17.0 (SPSS, Chicago, IL, USA). Using dose-volume histograms for the lung, the volume exposed to 5 Gy (RBE) or more (V5), V10 for 10 Gy (RBE), V15 for 15 Gy (RBE), V20 for 20 Gy (RBE), V25 for 25 Gy (RBE), V30 for 30 Gy (RBE), V40 for 40 Gy (RBE), and mean lung dose were calculated to evaluate the protocols.
Results
The median follow-up time was 33.7 months (range 4.6–57.5 months). The three-year OS, PFS, and LC rates were 81.3% (95% CI 75.9–86.7%), 73.4% (95% CI 67.2–79.6%), and 96.0% (95% CI 93.2–98.8%), respectively (). The patterns of recurrence were local progression in two patients, regional lymph node metastases in three patients, distant metastases in five patients, and carcinomatous pleuritis in two patients. Local failure was defined by an increase in FDG-PET uptake and CT-guided biopsy in two patients. One of the patients with SCC (T1a) treated with 66 Gy (RBE) in 10 fractions developed regional lymph node and liver metastases. Another patient with adenocarcinoma (T2a) treated with 80 Gy (RBE) in 25 fractions experienced local failure. One patient was diagnosed with a second primary NSCLC in the contralateral lung six months after the first PBT administration, and PBT was performed to treat the new lesion.
Figure 1. Local control, progression-free survival, and overall survival rates. LC, local control; OS, overall survival; PFS, progression-free survival.
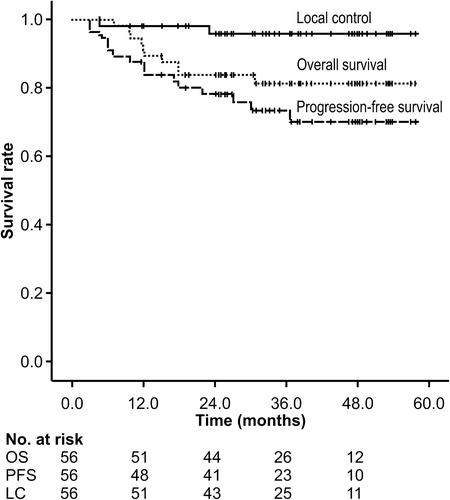
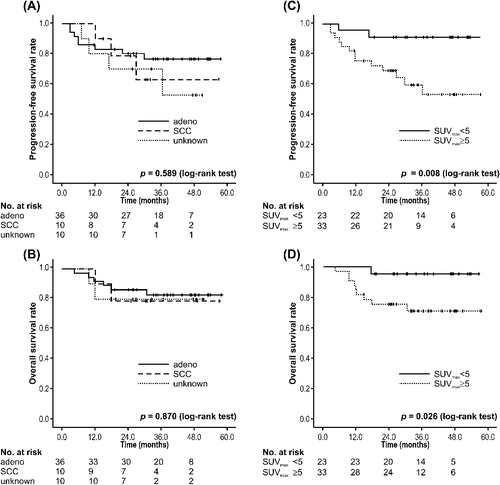
The PBT dose per fraction was not associated with LC (p = 0.902), PFS (p = 0.446), and OS (p = 0.789) (Supplementary Figure 1, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.948060). Sex, age (< 70 vs. ≥ 70 years), performance status (0 vs. 2 or 1), T-stage (T1a or T1b vs. T2a), and pathology (adenocarcinoma vs. SCC vs. unknown) were not associated with OS or PFS. Only SUVmax (SUVmax < 5 vs. SUVmax ≥ 5) was identified as a significant prognostic factor for OS (p = 0.008) and PFS (p = 0.026). The results of the prognostic factor analysis are shown in .
Table II. Univariate analysis of factors potentially affecting overall and progression-free survival.
No acute or late grade 4 and 5 toxicities were observed with either of the two protocols. The most common acute toxicity was dermatitis. One patient (1.8%) developed grade 3 dermatitis, and 10 patients (17.9%) developed grade 2 dermatitis. The most common late toxicity was rib fracture. Twenty patients (35.8%) developed rib fracture, but half of these patients (17.9%) were asymptomatic. One patient (1.8%) had grade 3 pneumonitis and was treated with oxygen and steroid therapy. Nine patients (16.1%) had grade 2 pneumonitis. Every case of radiation pneumonitis developed within eight months after PBT. One patient (1.8%) developed grade 2 pericardial effusion. The incidence of acute and late toxicities is summarized in .
Table III. Acute and late toxicities.
The outcomes for dosimetric parameters are shown in . The median PTV was 74.4 ml. Regarding lung tissue, the mean lung dose, V5, V10, V15, and V20, were 3.3 Gy (RBE), 10.7%, 8.7%, 7.2%, and 6.0% for protocol A, respectively, and 5.4 Gy (RBE), 13.2%, 12.3%, 10.2%, and 9.5% for protocol B, respectively.
Table IV. Dose volume analysis.
Discussion
The results of this study demonstrate the clinical outcomes of two PBT protocols for the treatment of NSCLC. Previous studies of PBT in early-stage NSCLC patients reported two-year LC rates of 86–95% using total doses of 51–87.5 Gy (dose per fraction 1.8–16 Gy) [Citation5–14] (Supplementary Table I, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.948060). Similar to these previous studies, the three-year LC rate was 96% among all patients treated in this study. In addition, two patients treated with BED 10 > 100 Gy (protocol A: 1 patient; protocol B: 1 patient) experienced local failure in this study. In a large retrospective study of SBRT in stage I NSCLC patients, Onishi et al. reported that BED 10 ≥ 100 Gy was associated with lower local failure incidence (8.4% vs. 42.9%) and superior OS (53.9% vs. 19.7% at 5 years) when compared with BED 10 of < 100 Gy [Citation15]. Therefore, the results of our study are roughly comparable to those using stereotactic hypofractionated irradiation of BED 10 ≥ 100 Gy.
Several SBRT series have reported reduced toxicity with high-dose fractionation in centrally and peripherally located tumors [Citation17,Citation18]. While few studies have included a long follow-up period after SBRT, there are currently several ongoing SBRT trials for centrally located tumors. Nakayama et al. reported that PBT with lower fraction doses may be advantageous in cases that involve central tumors [Citation10]. In their study, a total dose of 66 Gy in 10 fractions was administered to peripherally located tumors, and 72.6 Gy in 22 fractions (BED 10 of 96.6) was administered to centrally located tumors. With a median follow-up of 17.7 months, the OS, PFS, and LC rates at two years were 97.8%, 88.7%, and 97.0%, respectively. Chang et al. conducted a phase I/II study for stage I NSCLC with PBT at a total dose of 87.5 Gy (RBE) administered at 2.5 Gy (RBE) per fraction [Citation11]. With a median follow-up of 16.3 months (range 4.8–36.3 months), no patients experienced grade 4 or 5 toxicities, suggesting that this treatment protocol is an acceptable treatment option for patients with centrally located early-stage lung cancer. However, Bush et al. reported that for patients with T1 tumors, a regimen of 70 Gy in 10 fractions may serve as the standard approach when PBT is used for both central and peripheral tumor locations [Citation14]. We used a lower proton dose for challenging cases than that used in SBRT because some critical toxicities, such as aggravation of interstitial pneumonia or esophageal or gastric fistula, were reported with administration of SBRT [Citation1–3,Citation19]. In our study, a dose per fraction of 3.2 Gy (RBE) was used for tumors located near the mediastinum, proximal bronchial tree, and esophagus, and no serious treatment-related toxicities were observed.
Despite excellent LC, the OS rate for NSCLC has remained low. Chi et al. reviewed the patterns of failure after SBRT [Citation20]. Based on their report, chemotherapy should be considered for large tumors and centrally located lesions. In our study, there was no association between PBT dose and LC, PFS, and OS rates by univariate analysis. Although pathological diagnosis was not associated with OS and PFS, SUVmax (SUVmax < 5 vs. SUVmax ≥ 5) was identified as a significant prognostic factor for OS and PFS (). Recent studies have demonstrated that pretreatment SUVmax predicts clinical outcomes in patients with early-stage NSCLC treated with SBRT [Citation21,Citation22]. The International Association for the Study of Lung Cancer suggested that a higher SUVmax in NSCLC predicts poor prognoses [Citation23]. We also showed that SUVmax was a predictive factor associated with PFS and OS in stage I NSCLC treated with PBT. Thus, SUVmax may be used to identify candidates for adjuvant therapy after high-dose PBT.
Figure 2. Survival rates stratified by pathology and maximum standardized uptake value. (A) Progression-free survival and (B) overall survival stratified by tumor pathology. (C) Progression-free survival and (D) overall survival stratified by SUVmax < 5 or SUVmax ≥ 5. Adeno, adenocarcinoma; SCC, squamous cell carcinoma; SUVmax, maximum standardized uptake value.
Regarding acute toxicities, in a study by Chang et al., the most common adverse effect was dermatitis (grade 2: 67%; grade 3: 17%), followed by grade 2 fatigue (44%), grade 2 pneumonitis (11%), grade 2 esophagitis (6%), and grade 2 chest wall pain (6%). Fujii et al. reported grade 2 and 3 dermatitis in 14% and 4% of patients, respectively [Citation13]. In our study, we observed 21% incidence of grade > 2 dermatitis. Several studies have reported that proton therapy can result in clinically significant radiation dermatitis, and the risk of severe radiation dermatitis may limit beam arrangement and prescription doses [Citation24]. Regarding late toxicities, the rate of rib fractures was greater than that already reported. However, most of these fractures were associated with mild symptoms. The detection rate was attributed to the study method, which included rigorous evaluation of the ribs during the review of the CT scans. Kanemoto et al. reported dose-volume histogram parameters are useful in predicting late adverse events of rib fracture [Citation25]. Thus, dose constraints for the chest wall might be considerable. The incidence of radiation pneumonitis was comparable to that reported by Iwata et al. The toxicity was tolerable in both protocols in our study, similar to previous institutional reports; however, the incidence of toxicities, including dermatitis or rib fractures, may be further decreased by beam arrangement. Further investigation of the toxicities related to PBT is warranted.
The outcomes for dosimetric parameters, namely the mean lung dose, V5, and V10, were 3.3 Gy (RBE), 10.7%, and 8.7% for 66 Gy (RBE) in 10 fractions, and 5.4 Gy (RBE), 13.2%, and 12.3% for 80 Gy (RBE) in 25 fractions, respectively. Low-dose areas, such as V5 and V10, were completely reduced because of the characteristics of the proton beam. Kadoya et al. reported that dosimetric comparisons of protons and photons showed no differences between PBT and SBRT with regard to PTV conformity and large differences in V5 and V10 [Citation26]. Moreover, PBT was superior to SBRT when the PTV was more than 200 cm3. Thus, PBT appears to have an advantage over SBRT when the tumor is large, when treating a second tumor, or when treating several tumors because of its simple and excellent dose distribution.
Despite the limitations of the present study, including the small number of patients and retrospective study design, our PBT dose regimens seem to be reasonable for treatment of both centrally and peripherally located tumors. We confirmed the benefit in dose distribution to the lung and the tolerable toxicities. In addition, we also demonstrated a high LC rate and identified prognostic factors of high-dose PBT for stage I NSCLC.
In conclusion, here we have reported the toxicities and clinical outcomes of two PBT protocols for treatment of stage I NSCLC. Both PBT protocols achieved excellent LC with tolerable toxicities, and SUVmax was a significant prognostic factor after high-dose PBT.
Supplementary material available online
Supplementary Figure 1 and Table I, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.948060.
ionc_a_948060_sm9097.pdf
Download PDF (64.1 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833–9.
- Song SY, Choi W, Shin SS, Lee SW, Ahn SD, Kim JH, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009;66:89–93.
- Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, et al. Tolerance of organs at risk in small- volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys 2003;56:126–35.
- Register SP, Zhang X, Mohan R, Chang JY. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1015–22.
- Shioyama Y, Tokuuye K, Okumura T, Kagei K, Sugahara S, Ohara K, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:7–13.
- Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest 2004;126:1198–203.
- Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:107–11.
- Hata M, Tokuuye K, Kagei K, Sugahara S, Nakayama H, Fukumitsu N, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: Preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys 2007;68:786–93.
- Iwata H, Murakami M, Demizu Y, Miyawaki D, Terashima K, Niwa Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 2010;116:2476–85.
- Nakayama H, Sugahara S, Tokita M, Satoh H, Tsuboi K, Ishikawa S, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the university of tsukuba. Int J Radiat Oncol Biol Phys 2010;78:467–71.
- Chang JY, Komaki R, Wen HY, De Gracia B, Bluett JB, McAleer MF, et al. Toxicity and patterns of failure of adaptive/ablative proton therapy for early-stage, medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1350–7.
- Westover KD, Seco J, Adams JA, Lanuti M, Choi NC, Engelsman M, et al. Proton SBRT for medically inoperable stage I NSCLC. J Thorac Oncol 2012;7:1021–5.
- Fujii O, Demizu Y, Hashimoto N, Araya M, Takagi M, Terashima K, et al. A retrospective comparison of proton therapy and carbon ion therapy for stage I non-small cell lung cancer. Radiother Oncol 2013;109:32–7.
- Bush DA, Cheek G, Zaheer S, Wallen J, Mirshahidi H, Katerelos A, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: Results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys 2013;86:964–8.
- Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623–31.
- Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685–92.
- Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967–71.
- Milano MT, Chen Y, Katz AW, Philip A, Schell MC, Okunieff P. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol 2009;91:301–6.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677–82.
- Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: Clinical implications. Radiother Oncol 2010; 94:1–11.
- Nair VJ, MacRae R, Sirisegaram A, Pantarotto JR. Pretreatment [18F]-fluoro-2-deoxy-glucose positron emission tomography maximum standardized uptake value as predictor of distant metastasis in early-stage non-small cell lung cancer treated with definitive radiation therapy: Rethinking the role of positron emission tomography in personalizing treatment based on risk status. Int J Radiat Oncol Biol Phys 2014; 88:312–8.
- Satoh Y, Onishi H, Nambu A, Araki T. Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: Prognostic value. Radiology 2014;270:275–81.
- Paesmans M, Berghmans T, Dusart M, Garcia C, Hossein-Foucher C, Lafitte JJ, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: Update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol 2010;5:612–9.
- Kozak KR, Smith BL, Adams J, Kornmehl E, Katz A, Gadd M, et al. Accelerated partial-breast irradiation using proton beams: Initial clinical experience. Int J Radiat Oncol Biol Phys 2006;66:691–8.
- Kanemoto A, Mizumoto M, Okumura T, Takahashi H, Hashimoto T, Oshiro Y, et al. Dose-volume histogram analysis for risk factors of radiation-induced rib fracture after hypofractionated proton beam therapy for hepatocellular carcinoma. Acta Oncol 2013;52:538–44.
- Kadoya N, Obata Y, Kato T, Kagiya M, Nakamura T, Tomoda T, et al. Dose-volume comparison of proton radiotherapy and stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:1225–31.
