Abstract
Background. A contralateral tumor occurs in 3.5–5% of men diagnosed with testicular germ cell cancer (TGCC). Biopsy of the contralateral testis may detect intratubular germ cell neoplasia ITGCNU, a precursor of TGCC. Biopsy of the contralateral testis to detect ITGCNU is controversial. If adjuvant chemotherapy (ACT) protects against bilateral cancer is debated.
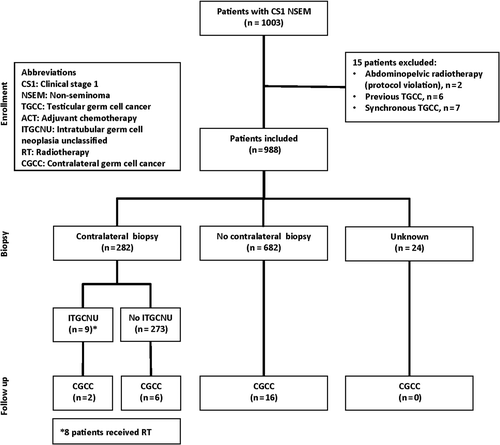
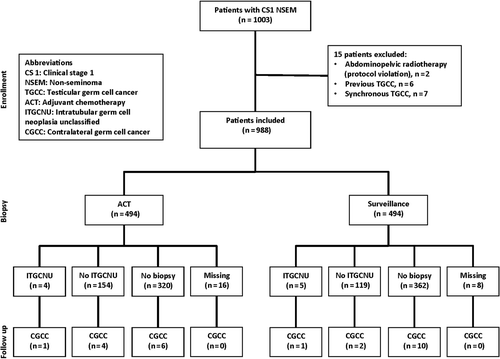
Material and methods. A total of 1003 patients with clinical stage I (CS I) non-seminomatous testicular germ cell cancer (NSGCT) were included in two prospective, population-based protocols. Fifteen patients were excluded. Treatment was either adjuvant chemotherapy (n = 494), or surveillance (n = 494). Contralateral testicular biopsy was recommended for all patients, but was performed only in 282 patients. In case of ITGCNU radiotherapy (RT) to 16 Gy was recommended.
Results. During a follow-up of 8.3 years, 31 (3.6%) patients developed contralateral TGCC. ITGCNU was detected in 3.2% (9/282) of biopsied patients. The incidence of bilateral TGCC was similar following ACT, 2.5% (11/494), and surveillance, 3.4% (13/494), p = 0.41. Young age was a risk factor for metachronous TGCC (HR 0.93; 95% CI 0.88–0.99, p = 0.02). In total 2.2% (6/273) of patients without ITGCNU in the biopsy developed contralateral TGCC. One irradiated patient developed contralateral cancer, and one developed contralateral tumor before RT was given.
Conclusion. ACT did not reduce the incidence of contralateral TGCC. Young patients had the highest risk of developing contralateral TGCC. The proportion of false negatives biopsies was higher than reported in earlier trials, but this may in part be related to patient selection, single biopsies and lack of mandatory immunohistochemistry.
Intratubular germ cell neoplasia of unclassified type (ITGCNU), synonymous to carcinoma in situ (CIS) or testicular intraepithelial neoplasia (TIN), is the precursor lesion of testicular germ cell germ cancer (TGCC). The reported incidence of biopsy-verified contralateral ITGCNU in unselected patient series with unilateral tumors is between 3.5% and 5% [Citation1,Citation2]. Age below 40 years, atrophic testes, cryptorchidism and sub-fertility confer a higher risk of ITGCNU [Citation3]. Untreated ITCGNU develops into an invasive testicular germ cell tumor (TGCC) in 50–70% of the patients within 5–7 years [Citation4,Citation5]. Synchronous or metachronous contralateral cancer is reported in about 3.5–5% of patients diagnosed with TGCC [Citation6–8]. The aim of performing a contralateral biopsy is to prevent a new TGCC by offering radiotherapy to eradicate confirmed ITGCNU. However, even patients with a negative biopsy may develop a contralateral tumor [Citation9,Citation10].
Norway and Sweden have age-adjusted TGCC incidence rates of 12.4/100 000 and 7.2/100 000, respectively [Citation11,Citation12]. Since 1981, The Swedish and Norwegian Testicular Cancer Group (SWENOTECA) has provided comprehensive management programs for testicular cancer. Recently we reported the clinical outcomes of risk-adapted treatment in clinical stage I (CS I) non-seminomatous germ cell testicular cancers (NSGCT) from the SWENOTECA III and VI management programs [Citation13,Citation14]. Both programs recommended a contralateral testis biopsy in order to diagnose ITGCNU. The aim of this study is to report the incidence of ITGCNU and bilateral TGCC in patients treated within the SWENOTECA III and VI programs according to biopsy status, and to evaluate the effect of adjuvant chemotherapy on the risk of developing a metachronous TGCC.
Patients and methods
The SWENOTECA III and VI programs included 1003 Swedish and Norwegian patients diagnosed with CS I NSGCT from July 1995 to July 2005. The data presented are population-based, and include all patients diagnosed with CS I NSGCT in Norway and Sweden within the timeframe of the studies, except patients treated at the Norwegian Radium Hospital.
The programs implemented adjuvant cisplatin-based chemotherapy, either in combination with vinblastine and bleomycin (CVB) or etoposide and bleomycin (BEP), based on a risk-adapted approach. Patients with lymphovascular invasion (LVI) of tumor cells in the primary tumor were recommended adjuvant chemotherapy, whereas patients without LVI had the choice of surveillance or adjuvant chemotherapy. The staging and follow-up procedures of these patients have been published earlier [Citation14]. Half of the patients (n = 502) were managed by surveillance, whereas the remaining 499 patients were given adjuvant chemotherapy. Alternative adjuvant treatment in the form of radiotherapy (n = 2 patients) and retroperitoneal lymph node dissection (RPLND) (n = 1) represented protocol violations. The patient treated with RPLND was classified as followed by surveillance with regard to risk of contralateral TGCC.
Biopsy of the contralateral testis was recommended, and identification of ITGCNU prompted adjuvant radiotherapy to a minimum dose of 16 Gy in 8 fractions.
The biopsy technique was described in the protocols:
The protocol stipulated a single biopsy to be taken and put in Bouin's solution if it was to be analyzed within 24 hours, otherwise in either Stieves fixative or formaldehyde.
The Swedish and Norwegian medical ethical committees approved the SWENOTECA management programs.
Statistical analysis
The incidence of metachronous contralateral cancer was estimated by using Kaplan-Meier survival curves. Time to development of a contralateral cancer was calculated from the date of orchiectomy for NSGCT to the diagnosis date of the second TGCC. Patient groups were compared using the log-rank test (surveillance vs. adjuvant chemotherapy and patients receiving a biopsy vs. unbiopsied patients). Possible risk factors for contralateral metachronous testicular cancer (LVI, adjuvant chemotherapy, age at orchiectomy and biopsy-status) were assessed in a univariable analysis using a Cox proportional hazards model. Variables with a p-value ≤ 0.25 in univariable analyses were planned entered in a multivariable Cox proportional hazards model. A p-value ≤ 0.05 was regarded as statistically significant. Except age, data on risk factors for ITGCNU were not registered in the database.
Results
Among the 1003 patients included in the protocols, six patients had previously been diagnosed with a contralateral TGCC tumor. Furthermore, seven patients presented with synchronous bilateral TGCC. Accordingly, 988 patients who underwent orchiectomy for CS1 NSGCT between July 1995 and July 2005 were still at risk of developing a contralateral cancer ().
Table I. Patients with previous metachronous or syncronous testicular cancer.
In most patients, 682/988 (69%), a contralateral biopsy was not performed. Only a minority of the patients, 282/988 (29%), underwent a contralateral biopsy, and biopsy status was unknown in 24/988 (2%) of the patients (). The proportion of patients biopsied among patients followed by surveillance and adjuvant chemotherapy was 25% and 32%, respectively ().
With a median follow-up of 8.3 years, 24 men were diagnosed with a metachronous TGCC (). The median time to metachronous TGCC was 3.7 years, with a range of 0.2–8.1 years. The incidence of bilateral cancers including synchronous TGCC, but excluding previous metachronous TGCC, was 3.6%.
Table II. Metachronous bilateral germ cell cancers.
ITGCNU was detected in 9/282 (3.2%) of patients undergoing a contralateral biopsy. Of the nine patients with ITGCNU, eight received radiotherapy. Two patients with confirmed ITGCNU developed metachronous TGCC. One patient developed a tumor shortly after diagnosis before radiotherapy was given, and most likely represented an undetected synchronous TGCC. The other patient developed a contralateral seminoma three years after radiotherapy (16 Gy) for ITGCNU. Seven patients treated with radiotherapy for ITGCNU never developed a contralateral TGCC.
Among 494 patients receiving adjuvant chemotherapy 74% received one and 16% two courses of adjuvant BEP/CVB. The incidence of bilateral cancer was not significantly different between patients managed by surveillance or treated with adjuvant chemotherapy, 3.4% and 2.5%, p = 0.41.
Only age at orchiectomy was a significant risk factor for developing metachronous cancer with a HR 0.93 (95% CI 0.88–0.99), p = 0.02 (), whereas none of the other proposed risk factors reached the threshold p-value for an association. Accordingly, we did not identify significant covariates for inclusion in multivariable analysis. The overall survival for patients developing contralateral cancer was 100%. The metachrounous TGCC presented as CS1 disease in 85% of the cases, and 70% of these were managed by surveillance only.
Table III. Possible prognostic factors, development of contralateral testicular cancer.
Discussion
In this study, approximately 4% of the patients with CS1 NSGCT developed a second testicular cancer, at a median of 3.7 years following orchiectomy. Short adjuvant cisplatin-based chemotherapy did not significantly reduce the risk of contralateral cancer in CS I NSGCT.
The excellent prognosis of metachronous TGCC found in this study is in accordance with several other reports [Citation15–17].
Treatment of contralateral ITGCNU will potentially spare patients from of a new testicular cancer diagnosis, as well as the burden of extensive treatment in the case of metastatic disease. In our study 15% of patients with a metachronous TGCC presented with metastatic disease. A new diagnosis of TGCC will extend the period of follow-up, and may also have impact on the individual's possibility to get a personal health insurance. For patients with confirmed ITGCNU planning to father children, it may be a difficult choice to determine whether to receive radiotherapy at diagnosis or to postpone the treatment with the risk of developing a second TGCC.
Successful screening for ITGCNU depends on the accurate detection of ITGCNU, if present. The theoretical basis for recommending a contralateral biopsy is the proposed uniform distribution of ITCGNU in the contralateral testis. A single biopsy only examines a very small part of the testis, and the accuracy is shown to increase with two biopsies. The reported discordance rate between two concomitant biopsies is 30%, and contradicts the notion of uniformly distributed ITGCNU [Citation18]. Even with three biopsies, 20% of the patients diagnosed with ITGCNU will only have one of three biopsies positive for ITGCNU [Citation19], proving that ITGCNU in many cases is focal, and not uniformly distributed. False negative biopsies will therefore always present a challenge.
Although data from the MRC TE19 trial indicate that the incidence of contralateral TGCC may be reduced in CS I seminomatous patients treated with adjuvant carboplatin [Citation20], it is possible that adjuvant carboplatin only postpones the development of contralateral TGCC [Citation21]. However, recent studies indicate that patients treated with cisplatin-based chemotherapy for metastatic disease may have a lower risk of developing contralateral testicular cancer [Citation8], and four or more courses of cisplatin-based chemotherapy seem to reduce the five-year probability of developing contralateral TGCC in patients with untreated ITGCNU [Citation5]. Our data, however, does not support a protective effect of short adjuvant cisplatin-based chemotherapy to prevent contralateral TGCC in CS I NSGCT.
In the present study, eight patients received adjuvant radiotherapy due to ITGCNU, and seven of these did not develop a contralateral cancer. Provided that a contralateral TGCC develops in 50–70% without adjuvant treatment, four to six patients were spared a second diagnosis of TGCC. With 282 patients biopsied, the number of patients needed to undergo biopsy to prevent one new contralateral TGCC was in the range of 47–70. Selected biopsies of young patients (< 40 years) with high-risk features only [sub-fertility, testicular atrophy (< 12 ml) or history of cryptorchidism] would probably reduce the number needed to undergo biopsy considerably [Citation3,Citation18]. Although data on complications from contralateral biopsy performed in these studies was not recorded, our experience is that the morbidity of the procedure is very low. This is also in accordance with a large study reporting a complication rate of 2.8%, with 0.6% of patients requiring repeated surgery [Citation22].
Radiotherapy in the case of ITGCNU will in most cases eradicate ITGCNU lesions. Unfortunately, these patients will be rendered infertile, and have a 50% risk of hypogonadism due to reduced Leydig cell function [Citation23]. The radiation dose has been reduced with the intention to preserve Leydig cell function. However, a treatment doses as low as 14 Gy have also resulted in impaired androgen production [Citation24]. As demonstrated in one patient in the present study, a radiation dose of 16 Gy may be insufficient to eradicate ITGCNU, and treatment failures have also been reported after 18 Gy testicular radiotherapy [Citation25]. However, the detection of ITGCNU may give the patient time to father a child while under surveillance with delayed radiotherapy, or to cryopreserve additional sperm.
Not surprisingly and in accordance with previous reports, young age was found to be a significant risk factor for metachronous cancer in this study [Citation3]. According to our findings, for each one-year increase in age at CS I NSGCT diagnosis, the risk of being diagnosed with a subsequent contralateral GCT is reduced by 7%. Young age is a known risk factor for ITCGNU [Citation1,Citation3], explaining the age-related association with contralateral tumor development observed in this study.
This study has some limitations. The incidence of metachronous TGCC is underestimated by the fact that most patients with ITGCNU would have developed metachronous TGCC without radiation therapy. If estimating that 70% of the patients with ITGCNU develop a contralateral TGCC, an additional six patients would have developed contralateral TGCC in this group.
A contralateral biopsy was performed only in a minority of patients (29%) and mostly in Sweden, although recommended in both protocols. The low biopsy frequency probably reflects the controversy of this recommendation, and most likely the biopsied patients were selected according to their risk profile of low age, sub-fertility, testicular atrophy or cryptorchidism. This is supported by the fact that 16/682 (2.3%) patients in the group not undergoing biopsy developed contralateral TGCC, compared to 14/282 (5%) in patients undergoing contralateral biopsy. The 14 patients are those eight patients that actually developed contralateral TGCC plus 70% (n = 6) of the patients with ITGCNU successfully treated with radiotherapy. Accordingly, more high-risk patients were offered a contralateral biopsy. The proportion of false negatives biopsies was high, but this may in part be related to single biopsies and lack of mandatory immunohistochemistry. As a consequence, it is difficult to make conclusions regarding the benefits from a recommendation of optimally performed biopsy of all.
SWENOTECA now recommends biopsy of the contralateral testis in all patients with risk factors. In all other patients we advocate patient autonomy. At the time of orchiectomy all patients should be informed of the possibility of contralateral biopsy. In patients who choose to undergo the procedure, a two-site biopsy should be taken, with mandatory immunohistochemical evaluation to reduce the risk of false negative results.
The evaluation of testicular biopsies requires an experienced pathologist. Orchiectomy is not an emergency surgical procedure, patients should be offered both cryopreservation of semen before orchiectomy and a testicular prosthesis and we therefore recommend that orchiectomy should be performed at a center with pathologists experienced in diagnosing ITGCNU and with access to cryopreservation and urologists with experience with implantation of a testicular prosthesis.
Except age, data on risk factors for ITGCNU were not registered in the database and are thus not included in the analyses. Our protocol did not include mandatory immunohistochemical staining and only a one-site biopsy was recommended. Large studies with mandatory immunohistochemical staining have reported up to 5% prevalence of ITGCNU [Citation1], whereas ITGCNU was detected in 3.2% of patients in our study. Moreover, patients with seminomas and metastatic NSGCT were not included. A major strength of this study is the prospective, population-based design. Patients were treated in a community-based manner according to binational management programs.
In conclusion, the prognosis for patients with bilateral testicular germ cell cancer within two comprehensive SWENOTECA management programs was excellent. Only a minority of patients underwent contralateral biopsy, and as such they represent a selected population. The proportion of false negatives was high, but this may in part be related to one-site biopsy and lack of mandatory immunohistochemistry. Our data does not support a protective effect of short adjuvant cisplatin-based chemotherapy to prevent contralateral TGCC in CS I NSGCT.
Acknowledgments
We thank all patients involved in the study. Investigators at the following hospitals participated in the study: Haukeland University Hospital, Bergen; Borås Hospital, Borås; Mälarsjukhuset Hospital, Eskilstuna; Gävle Hospital, Gävle; Sahlgrenska University Hospital, Göteborg; County Hospital Ryhov, Jönköping; Central Hospital, Karlstad; Linköping University Hospital, Linköping; Skåne University Hospital, Lund; Skåne University Hospital, Malmö; Oslo University Hospital, Oslo; Stavanger University Hospital, Stavanger; Danderyd Hospital, Stockholm; Karolinska University Hospital Solna, Stockholm; Karolinska University Hospital Södersjukhuset, Stockholm; Sundsvall Hospital, Sundsvall; University Hospital of North Norway, Tromsø; St. Olavs University Hospital, Trondheim; Umeå University Hospital, Umeå; Uppsala University Hospital, Uppsala; Västerås Hospital, Västerås; Växjö Central Hospital, Växjö; Ålesund Hospital, Ålesund; Örebro University Hospital, Örebro. We also thank all other hospitals participating in the SWENOTECAnetwork.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The SWENOETCA has in part been funded by the National Cancer Fund of Sweden.
References
- Dieckmann KP, Loy V. Prevalence of contralateral testicular intraepithelial neoplasia in patients with testicular germ cell neoplasms. J Clin Oncol 1996;14:3126–32.
- von der Maase H, Rorth M, Walbom-Jorgensen S, Sorensen BL, Christophersen IS, Hald T, et al. Carcinoma in situ of contralateral testis in patients with testicular germ cell cancer: Study of 27 cases in 500 patients. Br Med J (Clin Res Ed) 1986;293:1398–401.
- Harland SJ, Cook PA, Fossa SD, Horwich A, Mead GM, Parkinson MC, et al. Intratubular germ cell neoplasia of the contralateral testis in testicular cancer: Defining a high risk group. J Urol 1998;160:1353–7.
- Dieckmann KP, Skakkebaek NE. Carcinoma in situ of the testis: Review of biological and clinical features. Int J Cancer 1999;83:815–22.
- Brabrand S, Fossa SD, Cvancarova M, Axcrona U, Lehne G. Probability of metachronous testicular cancer in patients with biopsy-proven intratubular germ cell neoplasia depends on first-time treatment of germ cell cancer. J Clin Oncol 2012;30:4004–10.
- Bokemeyer C, Schmoll HJ, Schoffski P, Harstrick A, Bading M, Poliwoda H. Bilateral testicular tumours: Prevalence and clinical implications. Eur J Cancer 1993;29A:874–6.
- Osterlind A, Berthelsen JG, Abildgaard N, Hansen SO, Hjalgrim H, Johansen B, et al. Risk of bilateral testicular germ cell cancer in Denmark: 1960–1984. J Natl Cancer Inst 1991;83:1391–5.
- Andreassen KE, Grotmol T, Cvancarova MS, Johannesen TB, Fossa SD. Risk of metachronous contralateral testicular germ cell tumors: A population-based study of 7,102 Norwegian patients (1953–2007). Int J Cancer 2011;129:2867–74.
- Souchon R, Gertenbach U, Dieckmann KP, Hahn E, Ruwe M, Stambolis C, et al. Contralateral testicular cancer in spite of TIN-negative double biopsies and interval cisplatin chemotherapy. Strahlenther Onkol 2006;182:289–92.
- Dieckmann KP, Loy V. False-negative biopsies for the diagnosis of testicular intraepithelial neoplasia (TIN) – an update. Eur Urol 2003;43:516–21.
- Cancer Registry of Norway. Cancer in Norway 2010 – Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2012.
- Socialstyrelsen. Cancer incidence in Sweden 2011. Stockholm: Socialstyrelsen; 2012.
- Tandstad T, Cohn-Cedermark G, Dahl O, Stierner U, Cavallin-Stahl E, Bremnes RM, et al. Long-term follow-up after risk-adapted treatment in clinical stage 1 (CS1) nonseminomatous germ-cell testicular cancer (NSGCT) implementing adjuvant CVB chemotherapy. A SWENOTECA study. Ann Oncol 2010;21:1858–63.
- Tandstad T, Dahl O, Cohn-Cedermark G, Cavallin-Stahl E, Stierner U, Solberg A, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: The SWENOTECA management program. J Clin Oncol 2009;27:2122–8.
- Fossa SD, Chen J, Schonfeld SJ, McGlynn KA, McMaster ML, Gail MH, et al. Risk of contralateral testicular cancer: A population-based study of 29,515 U.S. men. J Natl Cancer Inst 2005;97:1056–66.
- Albers P, Goll A, Bierhoff E, Schoeneich G, Muller SC. Clinical course and histopathologic risk factor assessment in patients with bilateral testicular germ cell tumors. Urology 1999;54:714–8.
- Coogan CL, Foster RS, Simmons GR, Tognoni PG, Roth BJ, Donohue JP. Bilateral testicular tumors: Management and outcome in 21 patients. Cancer 1998;83:547–52.
- Dieckmann KP, Kulejewski M, Pichlmeier U, Loy V. Diagnosis of contralateral testicular intraepithelial neoplasia (TIN) in patients with testicular germ cell cancer: Systematic two-site biopsies are more sensitive than a single random biopsy. Eur Urol 2007;51:175–83; discussion 83–5.
- Kliesch S, Thomaidis T, Schutte B, Puhse G, Kater B, Roth S, et al. Update on the diagnostic safety for detection of testicular intraepithelial neoplasia (TIN). APMIS 2003;111:70–4; discussion 5.
- Oliver RT, Mead GM, Rustin GJ, Joffe JK, Aass N, Coleman R, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: Mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 Study (ISRCTN27163214). J Clin Oncol 2011;29: 957–62.
- Powles T, Robinson D, Shamash J, Moller H, Tranter N, Oliver T. The long-term risks of adjuvant carboplatin treatment for stage I seminoma of the testis. Ann Oncol 2008;19:443–7.
- Dieckmann KP, Heinemann V, Frey U, Pichlmeier U, German Testicular Cancer Study G. How harmful is contralateral testicular biopsy? – an analysis of serial imaging studies and a prospective evaluation of surgical complications. Eur Urol 2005;48:662–72.
- Petersen PM, Giwercman A, Hansen SW, Berthelsen JG, Daugaard G, Rorth M, et al. Impaired testicular function in patients with carcinoma-in-situ of the testis. J Clin Oncol 1999;17:173–9.
- Petersen PM, Giwercman A, Daugaard G, Rorth M, Petersen JH, Skakkeaek NE, et al. Effect of graded testicular doses of radiotherapy in patients treated for carcinoma- in-situ in the testis. J Clin Oncol 2002;20:1537–43.
- Dotsch M, Brauers A, Buttner R, Nolte-Ernsting C, Eble MJ, Jakse G. Malignant germ cell tumor of the contralateral testis after radiotherapy for testicular intraepithelial neoplasia. J Urol 2000;164:452–3.