Abstract
Background. Sarcopenia is a defining feature of cancer cachexia associated with physical decline, poor quality of life and poor prognosis. Thus, maintaining muscle mass is an important aim of cachexia treatment. Many patients at risk for developing cachexia or with cachexia experience side effects of chemotherapy that might aggravate the development of cachexia. However, achieving tumor control might reverse the catabolic processes causing cachexia. There is limited knowledge about muscle mass changes during chemotherapy or whether changes in muscle mass are associated with response to chemotherapy.
Patients and methods. In this pilot study, patients with advanced non-small cell lung cancer (NSCLC) receiving three courses of palliative chemotherapy were analyzed. Muscle mass was measured as skeletal muscle cross sectional area (SMCA) at the level of the third lumbar vertebrae using CT images taken before and after chemotherapy.
Results. In total 35 patients, 48% women, mean age 67 years (range 56–86), participated; 83% had stage IV disease and 71% were sarcopenic at baseline. Mean reduction in SMCA from pre- to post-chemotherapy was 4.6 cm2 (CI 95% −7.3–−1.9; p < 0.002), corresponding to a 1.4 kg loss of whole body muscle mass. Sixteen patients remained stable or gained SMCA. Of these, 14 (56%) responded to chemotherapy, while two progressed (p = 0.071). Maintaining or gaining SMCA resulted in longer median overall survival (loss: 5.8 months, stable/gain: 10.7 months; p = 0.073). Stage of disease (p = 0.003), treatment regimen (p = 0.023), response to chemotherapy (p = 0.007) and SMCA change (p = 0.040), but not sarcopenia at baseline, were significant prognostic factors in the multivariate survival analyses.
Conclusion. Almost half of the patients had stable or increased muscle mass during chemotherapy without receiving any cachexia treatment. Nearly all of these patients responded to the chemotherapy. Increase in muscle mass, but not sarcopenia at baseline, was a significant prognostic factor.
Cancer cachexia is a common feature of advanced cancer, and has been estimated to be the main cause of death in 20% of cancer patients [Citation1]. The syndrome is characterized by anorexia, reduced food intake, metabolic changes, weight loss, low body mass index and/or low skeletal muscle mass (sarcopenia) [Citation2,Citation3]. Recent studies show that sarcopenia is frequent in advanced cancer, and is associated with physical decline, reduced quality of life, increased chemotherapy toxicity and shorter survival time [Citation4–8].
Muscle mass can be assessed from CT images and has been proposed as an important entry criteria and outcome for clinical trials of cachexia therapy [Citation9]. Studies using CT image analysis to measure muscle mass, have revealed a loss of muscle mass during anti-cancer therapy in patients with various advanced cancers [Citation7,Citation10–14], but some of them also show that patients might gain muscle mass [Citation7,Citation13,Citation14]. Catabolic processes, such as inflammation (associated with high C-reactive protein and low albumin values [Citation15]), abnormal metabolism and reduced caloric intake due to the underlying malignancy are considered to be main causes of muscle loss [Citation14]. Thus, it has been proposed that successful treatment of the underlying malignancy can reduce or even reverse the catabolic effects on the muscle mass [Citation7].
Although it has been shown that chemotherapy might improve quality of life and survival in advanced cancer patients [Citation16], there is still limited knowledge about changes in muscle mass during chemotherapy in patients with advanced cancer, and whether changes in muscle mass are associated with response to the treatment.
Non-small cell lung cancer (NSCLC) is the most common cause of cancer-related deaths. Nearly all patients develop advanced, incurable disease for which palliative chemotherapy is the recommended therapy. The prevalence of sarcopenia is high (40–60%) and higher than in most other types of cancer [Citation4]. Thus, in this study of patients with advanced NSCLC receiving three cycles of platinum-based chemotherapy, the primary aim was to explore changes in muscle mass assessed by CT images taken before and after chemotherapy in order to answer the following research questions:
Does skeletal muscle mass change during palliative chemotherapy?
Are there any associations between changes in muscle mass and response to chemotherapy?
As a secondary aim, we explored whether change muscle is an independent prognostic factor for survival.
Methods
This pilot observational cohort study used data from a randomized study comparing quality of life during chemotherapy with two different regimens for advanced NSCLC [Citation17]. Main eligibility criteria were written informed consent, age ≥ 18 years, WHO performance status score (PS) 0–2, and stage IIIB–IV NSCLC eligible for palliative chemotherapy [Citation17]. Patients were randomized to receive either carboplatin AUC = 5 (Calvert's formula) day 1 plus vinorelbine 25 mg/m2 day 1 & 8 or carboplatin AUC = 5 (Calvert's) day 1 plus gemcitabine 1000 mg/m2 day 1 & 8 every three weeks. Three courses were planned for all patients.
Patients who received at least one course of chemotherapy and had a CT scan before and after chemotherapy were included in the present study. The study was approved by the Regional Committee for Medical and Health Research Ethics in Central Norway.
Imaging and assessment of skeletal muscle mass
CT scans were taken within two weeks before chemotherapy commenced and within three weeks after the last course of chemotherapy was administered. Median time between the CT scans was 88 days (SD 22; range 43–122).
Muscle mass was measured as total SMCA using CT-images at the third lumbar vertebra level (L3) and expressed in cm2 [Citation18]. The Slice O’ Matic v 4.3 by Tomovision, Canada software was used for image analysis. One image (with a maximum slice thickness of 5 mm) was selected for each patient. During anatomical land marking, the first image at L3 with both vertebral transverse processes clearly visible, were used in the analysis. Radio density of the skeletal muscle was calculated by use of Hounsfield Units, with thresholds from − 29 to + 150 [Citation18].
Change in SMCA was dichotomized according to change from pre-to post-chemotherapy: 1) patients with > 2% loss of SMCA; and 2) all other patients. The cut-offs were defined according to the previously reported measurement error of 2% for CT image analysis at the L3 vertebral level [Citation19], equivalent to a change of whole body skeletal muscle mass of ≥ 1 kg [Citation20]. Whole body muscle mass in kilogram was calculated from the SMCA, as described by Mourtzakis et al. [Citation19]. Thresholds for classifying sarcopenia at baseline were based on previously reported cut-off values; SMCA normalized for stature (height, m2) ≤ 38, 5 cm2/m2 for women and ≤ 52, 4 cm2/m2 for men [Citation21].
Other assessments
Stage of disease was assessed at baseline according to the TNM v7.0 [Citation22]. Response to chemotherapy was assessed by comparing the post-treatment CT scans with pre-treatment images and was classified according to the RECIST-criteria v1.1 as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) [Citation23]. Toxicity was classified and graded according to the CTCAE 3.0 [Citation24]. Weight loss (kg) the last three months before start of chemotherapy was assessed by patients’ self-report. Values of C-reactive protein (CRP) and albumin were collected from hospital medical records. Patients reported health-related quality of life (HRQoL) at pre-treatment and three weeks after the last course of chemotherapy by the European Organization for Research and Treatment of Cancer (EORTC) Quality of life Questionnaires C30 and LC 13 [Citation25].
Data analysis and statistical analysis
Descriptive data is presented as means and standard deviations (SD) for continuous variables and proportions and percentages for categorical variables. χ2 and Fisher's exact tests were used for group- comparisons in categorical variables, and for the purpose of statistical analysis variables were dichotomized. Thus, patients with a CR, PR or SD were categorized as having disease-control, and those with progressive disease as having progression.
Survival time was defined as time from inclusion in the study until death and was estimated using the Kaplan-Meier method. The log-rank test was used for uni-variate survival analysis and the Cox proportional hazard method for multivariate survival analysis, adjusting for established prognostic factors in advanced NSCLC (PS, stage of disease, gender, tumor response, patient-reported global quality of life and appetite loss at baseline; and weight loss), CRP- and albumin-values at baseline, sarcopenia at baseline, body mass index and treatment [Citation5,Citation26].
Results
Patient characteristics
From August 2009 to May 2010, 54 patients were enrolled in the main study. Of these, 35 patients were eligible for inclusion in the present study. Reasons for exclusion were discontinuation from the main trial or death (n = 10), or no CT scans at the L3 vertebral level both before and after chemotherapy (n = 9).
Patients characteristics are shown in . Median age was 66 (range 55–86) years, 18 (51%) were men, 29 (83%) had stage IV disease, 4 (11%) had PS 0 and 29 (83%) had PS 1. Mean body weight was 71.5 kg (SD 14.4); 2 (6%) were underweight (BMI < 18.5 kg/m2), 17 (49%) had normal weight (BMI 18.5–24.9 kg/m2), 13 (37%) were overweight (BMI 25.0–29.9 kg/m2), and three (9%) were obese (BMI > 30.0 kg/m2). Mean weight loss during the three months prior to chemotherapy was 3.4 (SD 4.5) kg, 21 (60%) had < 5% loss of body weight, eight (26%) between 5% and 10%, and five (14%) more than 10%. Twenty-six (74%) patients, 12 women and 14 men, were sarcopenic at baseline.
Table 1. Baseline characteristics.
Changes in skeletal muscle mass
Mean SMCA reduced in the total cohort from 121.9 cm2 to 117.4 cm2 from pre- to post chemotherapy (mean change 4.6 cm2, CI 95% −7.3–−1.9; p < 0.002) , corresponding to a reduction of whole body muscle mass of 1.4 kg (CI 95% 0.6–2.2) [Citation19]. Nineteen (54%) had a reduction in SMCA, with a mean decrease of 10.3 cm2 (CI 95% −13.1–−7.4; p < 0.001). Sixteen patients (46%) had stable or increased SMCA, with a mean increase of 2.2 cm2 (CI 95% 0.5–3.9; p = 0.016). shows that amongst patients who were sarcopenic at baseline, 14/26 (54%) had stable or increased muscle mass, whereas among non-sarcopenic patients, the corresponding numbers were 2/9 (22%) (p = 0.104).
Figure 1. Change in skeletal muscle cross sectional area in patients according to sarcopenia. Individual changes in skeletal muscle cross sectional area (SMCA) from pre- to post-chemotherapy (Y-axis) according to sarcopenia at baseline, measured in cm2. Negative values indicate loss in muscle mass. Thresholds for sarcopenia were based on previously reported cut-off values for the skeletal muscle index, which were SMCA/body height 2; ≤ 38.5 cm2/m2 for women and ≤ 52.4 cm2/m2 for men [Citation20]. Non-sarcopenic patients (n=9) are shown in the bars to the left, and sarcopenic (n=26) in the bars to the right. Black bars are SMCA Loss, Dotted grey bars are SMCA Stable and Light grey bars are SMCA Gain.
![Figure 1. Change in skeletal muscle cross sectional area in patients according to sarcopenia. Individual changes in skeletal muscle cross sectional area (SMCA) from pre- to post-chemotherapy (Y-axis) according to sarcopenia at baseline, measured in cm2. Negative values indicate loss in muscle mass. Thresholds for sarcopenia were based on previously reported cut-off values for the skeletal muscle index, which were SMCA/body height 2; ≤ 38.5 cm2/m2 for women and ≤ 52.4 cm2/m2 for men [Citation20]. Non-sarcopenic patients (n=9) are shown in the bars to the left, and sarcopenic (n=26) in the bars to the right. Black bars are SMCA Loss, Dotted grey bars are SMCA Stable and Light grey bars are SMCA Gain.](/cms/asset/2c0d6b42-e06a-4f68-92dc-1998e08f11e3/ionc_a_953259_f0001_b.gif)
Associations between response to chemotherapy, laboratory values, changes in skeletal muscle mass and changes in HRQoL
A total of 25 patients (71%) had disease-control following chemotherapy (response or stable disease), while 10 patients (29%) progressed (). Among those with disease-control, 14/25 (56%) maintained or gained SMCA, whereas 2/10 (20%) of those who progressed gained muscle mass (p = 0.071). Descriptive data of changes in SMCA from pre- to post-chemotherapy depending on response to the chemotherapy are illustrated in .
Figure 2. Change in skeletal muscle cross sectional area in patients according to response to treatment. (Includes Figure A, B and C). Individual changes in skeletal muscle cross sectional area (SMCA) from pre- to post-chemotherapy (Y-axis) according to response to treatment, measured in cm2. Negative values indicate loss in muscle mass. Black bars are SMCA Loss, Dotted gray bars are SMCA Stable and Light gray bars are SMCA Gain.
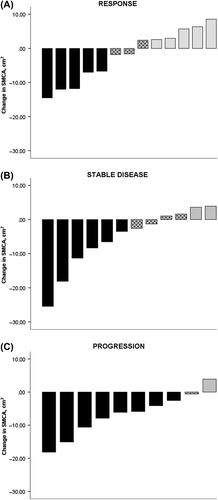
Table II. Courses of chemotherapy, response evaluation and toxicity.
CRP or albumin levels at the start of chemotherapy were not significantly associated with change in muscle mass. Among patients with a CRP ≥ 10 ml/g, there were 14 patients who maintained or gained SMCA and 12 who lost SMCA (p = 0.101). For albumin, the proportions with low values (< 36 m/l) were 6/14 and 4/12 before and after chemotherapy, respectively (p = 0.283).
Patients who maintained or gained SMCA, had an increase in physical function, with a change in mean scores from 65.7 (SD 22.2) to 66.2 (SD 20.2) points; for reduced appetite loss, the mean score changed from 19.0 (SD 25.2) to 14.3 (SD 31.3), while those with disease progression had a decline in physical function [mean score changed from 65.3 (SD 28.8) to 61.8 (SD 27.7) and increase in appetite loss (mean score changed from 20.0 (SD 27.6) to 22.2 (SD 32.5)].
Toxicity
Neutropenia (n = 28) and thrombocytopenia (n = 13) were the most common grade 3–4 toxicities (). Those who were sarcopenic at baseline did not experience more toxicity from the chemotherapy. There were no significant differences in grade 3–4 toxicity between patients who maintained/gained or those who lost SMCA.
Survival
In the uni-variate analysis, stage of disease (IIIB: 18.6 months vs. IV: 7.4 months; p = 0.034), treatment response (disease-control: 10.7 months vs. progression: 4.1 months; p < 0.001) and appetite loss (no appetite loss: 10.7 months vs. appetite loss: 6.7 months; p = 0.022) were significant prognostic factors for survival (). There was a trend towards shorter survival for patients who lost SMCA compared to patients with stable or gained SMCA (5.8 vs. 10.7 months, p = 0.073). Sarcopenia at baseline was not a significant prognostic factor (non-sarcopenic: 7.9 vs. sarcopenic 7.5 months; p = 0.490).
Table III. Uni- and multivariate survival analysis.
Stage of disease (p = 0.003), treatment regimen (p = 0.023), response to treatment (p = 0.007) and stable/gain in SMCA (p = 0.040) were significant prognostic factors in the multivariate survival analysis.
Discussion
In this exploratory pilot study of patients with advanced NSCLC we found a mean reduction in muscle mass of 1.4 kg during nine weeks of first-line platinum-doublet chemotherapy. There were large variations in changes of SMCA corresponding to changes in whole body muscle mass from −7.6 to + 2.6 kg. Furthermore, there was a trend towards less loss of muscle mass among those with disease control compared with the patients who progressed during the chemotherapy. Despite the absence of specific cachexia therapy (e.g. nutritional support, anti-inflammatory medication or physical exercise), 46% of patients had a stable or increased muscle mass following chemotherapy. These patients also had a slight improvement in self-reported physical function and appetite, and significantly longer survival compared to other patients. The response rate to chemotherapy was similar as in other studies of NSCLC [Citation27], and established prognostic factors in advanced NSCLC (stage of disease, loss of appetite and response to treatment) but not sarcopenia pre-chemotherapy were significant prognostic factors for time to death.
Maintained or gained muscle mass during cytotoxic chemotherapy has to our knowledge only been demonstrated in one other study. In that study, patients with various types of advanced cancer were included [Citation7]. Similar to our study, large variations in changes of muscle mass during systemic therapy have been found in other studies of NSCLC [Citation10] and advanced pancreatic cancer [Citation11,Citation13]. In the study by Murphy et al, NSCLC patients lost 1.1 kg of skeletal muscle over the duration of chemotherapy, but the mean change in muscle mass ranged from a loss of −6. 9 kg/100 days to a gain of + 1.6 kg/100 days [Citation10]. In both studies of advanced pancreatic cancer patients, muscle loss was predominant, but Tan et al. showed that 14% of the patients had a mean muscle gain of 7.9 ± 14.4%/100 days [Citation13], and in the study by Dalal et al., 34% of the patients gained muscle mass [Citation11].
None of these studies did however investigate whether changes in muscle mass were associated with response to the systemic therapy. Nevertheless, a link between gain in muscle mass and stable disease has been proposed by Prado et al. [Citation7]. They found that 15% of patient with advanced cancer gained muscle mass and nearly 50% remained stable over the clinical course of cancer disease. Patients with large gains in muscle mass had better response to treatment, ate well and had good symptom control, whereas those who lost muscle mass, had progressive disease and a short survival [Citation7]. In our study, we found that almost all of those who maintained or increased muscle mass had stable disease or responded to the chemotherapy. Thus, it appears that the chemotherapy might suppress the catabolic processes driving muscle breakdown in those who respond to the treatment – since no specific interventions aiming at preventing or reversing cachexia was administered (e.g. anti-inflammatory medication, nutritional support or physical exercise). It is noteworthy that our patients received a relatively short chemotherapy regimen.
It should be acknowledged that there are multiple causes of muscle loss in advanced cancer. In addition to the catabolic effects on the muscle caused by the underlying malignancy and cachexia, advanced cancer patients often have a reduced caloric intake and are physically inactive, which adds to the muscle loss. In addition, nausea, vomiting and loss of appetite are well-known side effects of chemotherapy that might negatively influence energy intake and activity levels and thus contribute to aggravate loss of muscle mass. These side effects are frequently reported among patients receiving the regimens administered in our study. There were no differences in grade 3–4 toxicity between those with stable/increased or those who lost muscle mass among our patients.
We are aware of three other studies in patients with advanced pancreatic cancer investigating whether change in muscle mass during the course of cancer treatment as a prognostic factor for survival [Citation11–13]. Unlike our study, neither of these studies demonstrated a statistically significant difference in survival related to change in muscle mass, possibly due to small sample sizes. Another likely explanation could be that methods for assessing muscle mass in these studies were different from the methods we used. We used previously reported cut-off values in the assessment of sarcopenia and for calculating longitudinal changes in muscle mass during chemotherapy. Still, the diversity of methods makes comparison between studies challenging and future studies should therefore address cut-offs for this outcome.
Our finding, that sarcopenia at baseline was not a significant prognostic factor for survival is not in line with previous studies conducted in larger cohorts of advanced cancer patients. The short expected survival of patients with advanced NSCLC and the high proportion of sarcopenic patients in our study (> 70%) are possible explanations. An association between sarcopenia and chemotherapy toxicity has been observed previously [Citation8]; the finding were however not replicated in our study cohort.
The main limitation of our study was the small sample size, limiting the power of the survival analyses. However, the sample is, in our opinion, large enough to demonstrate that more knowledge is needed before the role of assessing muscle mass in advanced cancer patients can be established – as a prognostic factor as well as in research on classification and treatment of cachexia. Another limitation is the use of cut-off levels for the definition of sarcopenia – there might be differences in body composition between countries. In case, this might influence the survival analyses, but not the analyses of changes in muscle mass.
Conclusion and suggestions for future research
In this exploratory study of patients with advanced NSCLC, we found large individual variations in changes in muscle mass during palliative chemotherapy. We observed that many patients had an increase in muscle mass without receiving any additional cachexia therapy and that many of these patients were sarcopenic before starting chemotherapy. There was also a trend towards more gain in muscle mass among patients who had disease control following chemotherapy, suggesting that response to cancer treatment is essential for controlling or reversing cancer cachexia. The finding that change in SMCA was a significant prognostic factor for survival, suggest that response to cancer therapy and changes in SMCA, and not only muscle mass measured before starting chemotherapy, should be assessed in future studies on prognosis of cancer cachexia. Furthermore, use of SMCA as an endpoint in studies of cancer cachexia should be used with caution and need careful consideration until more is known about changes in muscle mass in patients undergoing anti-cancer therapy.
Key message
Sarcopenia or loss of skeletal muscle mass has recently been proposed as a key clinical feature of cachexia. Assessment of skeletal muscle mass on CT images might be used to classify cachexia and to evaluate the effect of interventions aiming at controlling or reversing cachexia. However, little is known about the nature and magnitude of changes in muscle mass in cancer patient during the course of their disease – or during cancer therapy. In our pilot study of patients with advanced non-small cell lung cancer, nearly 50% of the patients had stable or increased muscle mass during chemotherapy without receiving any cachexia treatment. Almost all of these patients responded to the chemotherapy – suggesting that tumour control is essential for successfully treating cachexia. Furthermore, change in muscle mass during treatment and not sarcopenia presenting at baseline, was a significant prognostic factor for survival. More studies about longitudinal changes in skeletal muscle mass in cancer patients – and the correlation with response to cancer therapy – are needed before the role of sarcopenia in advanced cancer and the value in cachexia research can be established.
Acknowledgements
The European Centre for Palliative Care Research and the Department of Cancer Research and Molecular Medicine, NTNU. This work is part of a PhD degree supported by Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: Facts and numbers. J Cachexia Sarcopenia Muscle 2010;1:1–5.
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: A new definition. Clin Nutr 2008;27:793–9.
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489–95.
- Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 2010;91:1133S–7S.
- Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–47.
- Parsons HA, Baracos VE, Dhillon N, Hong DS, Kurzrock R. Body composition, symptoms, and survival in advanced cancer patients referred to a phase I service. PLoS One 2012;7:e29330.
- Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, et al. Central tenet of cancer cachexia therapy: Do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr [Internet]. [cited 2013 Aug 21]. Available from: http://ajcn.nutrition.org/content/98/4/1012.full.pdf.
- Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care 2013;7:383–9.
- Baracos V, Caserotti P, Earthman CP, Fields D, Gallagher D, Hall KD, et al. Advances in the science and application of body composition measurement. J Parenter Enteral Nutr 2012;36:96–107.
- Murphy RA, Mourtzakis M, Chu QS, Reiman T, Mazurak VC. Skeletal muscle depletion is associated with reduced plasma (n-3) fatty acids in non-small cell lung cancer patients. J Nutr 2010;140:1602–6.
- Dalal S, Hui D, Bidaut L, Lem K, Del Fabbro E, Crane C, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: A pilot study. J Pain Symptom Manage 2012;44:181–91.
- Di Sebastiano KM, Yang L, Zbuk K, Wong RK, Chow T, Koff D, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br J Nutr 2013;109 :302–12.
- Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–9.
- Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr 2009;89:1173–9.
- Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer 2002;87:264–7.
- Mannion E, Gilmartin JJ, Donnellan P, Keane M, Waldron D. Effect of chemotherapy on quality of life in patients with non-small cell lung cancer. Support Care Cancer 2014;22:1417–28.
- Gronberg BH, Sørhaug S, Hjelde HH, Stene GB, Amundsen T, editor. Variation in health-related quality of life (HRQoL) during chemotherapy for advanced non- small cell lung cancer. 2013 ASCO Annual Meeting; 2013; Chicago, Illinois, USA. J Clin Oncol 2013;31 (Suppl; abstr 9562).
- Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 2009;3:269–75.
- Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006.
- Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97:2333–8.
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol 2008;9:629–35.
- Sobin LHG, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours, 7th ed. Wiley-Blackwell; Oxford. 2009.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality- of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Gronberg BH, Sundstrom S, Kaasa S, Bremnes RM, Flotten O, Amundsen T, et al. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer 2010;46:2225–34.
- Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285–91.
