Abstract
Background. For stage II and III head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy alone, loco-regional recurrence is the main cause of treatment failure. Strategies to improve loco-regional control should not be at the expense of increased late normal tissue toxicity. We investigated dose-intensified hypofractionated intensity-modulated radiotherapy (IMRT) with synchronous cetuximab.
Material and methods. In a phase I/II trial, 27 patients with stage III or high risk stage II HNSCC were recruited. They received three dose level simultaneous integrated boost IMRT, 62.5 Gy in 25 daily fractions to planning target volume one over five weeks with synchronous cetuximab. The primary endpoint was acute toxicity. Secondary endpoints included: late toxicity and quality of life; loco-regional control, cause-specific and overall survival.
Results. Radiotherapy was completed by 26/27 patients; for one (4%) the final fraction was omitted due to skin toxicity. All cycles of cetuximab were received by 23/27 patients. Grade 3 acute toxicities included: pain (81%), oral mucositis (78%) and dysphagia (41%). There were few grade 3 physician-recorded late toxicities, including: pain (11%), problems with teeth (8%) and weight loss (4%). At 12 months, only one (4%) patient required a feeding tube, inserted prior to treatment due to dysphagia. The maximal/peak rates of patient-reported late toxicities included: severe pain (11%), any dry mouth (89%) and swallowing dysfunction that required a soft/liquid diet (23%). At 12 months, all quality of life and most symptoms mean scores had resolved to baseline or were only a little worse; dry mouth, sticky saliva and dentition scores remained very much worse. At a median follow-up of 47 months, there were five (18.5%) loco-regional recurrences and the overall cause-specific survival was 79% (95% CI 53–92).
Conclusions. This regimen is safe with acceptable acute toxicity, low rates of late toxicity and impact on quality of life at 12 months following treatment. Further evaluation is recommended.
For stage II and III head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy alone, loco-regional recurrence is the main cause of treatment failure [Citation1]. This is associated with a reduction in overall survival [Citation2]. For this group of patients, there is therefore a need to investigate strategies that improve loco-regional tumour control, including use of synchronous systemic therapies and radiation intensification (dose escalation and/or altered fractionation) [Citation3,Citation4]. However, this should not be at the expense of increased late normal tissue toxicity.
In a meta-analysis of 50 trials including a total of 9615 patients, the addition of synchronous chemotherapy to radiotherapy for locally advanced HNSCC resulted in an overall survival benefit of 6.5% at five years [Citation3]. However, this is associated with increased late toxicity and questionable improvement in the therapeutic ratio. In a meta-analysis of three synchronous chemotherapy radiotherapy trials (two employing conventional fractionation and one an accelerated concomitant boost radiotherapy schedule), 43% of the 230 patients developed severe late toxicity [Citation5]. The development of severe and enduring swallowing dysfunction is of particular concern. It has the greatest impact on quality-of-life and risks potentially life threatening sequelae including aspiration pneumonia [Citation6,Citation7]. In one study, 45% of patients who received synchronous treatment required prolonged tube feeding or repeated pharyngeal or oesophageal dilatation [Citation7].
Cetuximab is an IgG1 monoclonal antibody that targets and inhibits the epidermal growth factor receptor (EGFR). In the landmark Bonner trial, the addition of cetuximab to radiotherapy for locally advanced HNSCC improved five-year overall survival rates compared with radiotherapy alone (45.6% vs. 36.4%), without increased late toxicity [Citation8]. Provisional data from a phase II/III trial of 421 patients with locally advanced HNSCC reported similar survival outcomes for use of synchronous cetuximab or cisplatin [Citation9]. However, results from a retrospective series of 174 patients suggested inferior effectiveness with cetuximab [Citation10]. There are no known predictive biomarkers for response to cetuximab. Whether patients with an improved prognosis [e.g. intermediate stage or human papilloma virus (HPV)-associated disease] may benefit from substitution of synchronous chemotherapy with cetuximab is uncertain.
Radiotherapy acceleration reduces the effect of tumour repopulation. Hypofractionated schedules accelerate treatment by employing fewer but larger radiation fractions (> 2.0 Gy per fraction). In a meta-analysis, treatment acceleration without total dose reduction (a category including eight trials and 3818 patients) increased five-year locoregional tumour control by 7.3%, but there was no overall survival benefit [Citation4]. Some schedules were associated with an unacceptable increase in acute toxicity and consequential late effects [Citation11,Citation12]. This is partly due to the use of conventional 2D-planned radiotherapy and should be improved by application of highly conformed volumes, using intensity-modulated radiotherapy (IMRT). In the meta-analysis, treatment acceleration with a reduction in the total dose did not improve locoregional tumour control [Citation4]. However, more than half of patients in this group received the CHART regimen (54 Gy in 36 fractions over 12 days), which can be criticised for an ‘overshortening’ of the overall treatment time, where the relative onset of accelerated repopulation between normal mucosa (seven days) and tumour (21 days) was not fully exploited [Citation13]. For five fractions per week, the modelled optimal overall treatment time (to match early and late biologically equivalent doses to obtain greatest tumour effect) is in the range 20–32 days (approx. 3–5 weeks) [Citation14].
There are no established local tumour control or survival benefits from accelerated compared with conventionally fractionated treatments when combined with synchronous chemotherapy. In RTOG 0129, there were no differences in local control or survival between conventional (70 Gy over seven weeks) or accelerated concomitant boost radiotherapy (72 Gy over six weeks) with cisplatin chemotherapy [Citation15]. The Groupe d’Oncologie Radiothérapie Tête Et Cou (GORTEC) 99-02 trial compared conventional radiotherapy with synchronous chemotherapy (70 Gy over seven weeks plus three cycles of four days’ carboplatin-fluorouracil chemotherapy), accelerated radiotherapy with synchronous chemotherapy (70 Gy in six weeks plus two cycles of five days’ carboplatin-fluorouracil) or very accelerated radiotherapy alone (64.8 Gy, 1.8 Gy twice daily in three and a half weeks) [Citation16]. Most patients had stage IV disease, 90% T3–T4 disease and 79% N1–N3 disease. Conventional radiotherapy with synchronous chemotherapy improved overall survival compared with very accelerated radiotherapy alone and there was no difference in overall survival between the accelerated or conventional fractionated radiotherapy with synchronous chemotherapy groups.
This lack of observed incremental benefit may be due to patient selection. The DAHANCA-6&7 trial showed modest treatment acceleration improved primary tumour rather than nodal disease site control [Citation17]. Similarly, in a Cochrane review, the effect of altered fractionation (hyperfractionation or acceleration) was significantly more pronounced on the primary tumour than nodal disease [Citation18]. The treatment effect on loco-regional failure was superior for N0 and N1 than N2 or N3 disease. Furthermore, the effect of a reduction in overall treatment time and impact on repopulation may be reduced by tumour dedifferentiation [Citation19]. For patients with small tumour bulk (T1, T2 and small T3) and N0 or N1 disease, accelerated radiotherapy without chemotherapy is an appropriate regimen [Citation1]. There are only limited data to assess the addition of cetuximab to altered fractionated radiotherapy. However, in the Bonner trial over half of patients received a concomitant boost schedule and in sub-group analysis, the effect of cetuximab was more pronounced in this group compared with conventional fractionation [Citation8].
In those with intermediate stage disease, the addition of cetuximab to accelerated fractionated radiotherapy has the potential to improve treatment effectiveness without increased late toxicity, i.e. optimise the therapeutic ratio for this group of patients. In a phase I/II trial, we investigated dose-intensified hypofractionated IMRT with 62.5 Gy in 25 daily fractions over five weeks (2.5 Gy per fraction; biologically equivalent dose to the tumour, 70.9 Gy10 and late reacting tissues, 114.6 Gy3) [Citation20] with synchronous cetuximab in patients with Union for International Cancer Control (UICC) stage III (T1–3N1 or T3N0) or high risk stage II (T2N0, excluding glottic laryngeal) disease.
Material and methods
Study design
This was a phase I/II radiotherapy study of dose intensified hypofractionated IMRT with synchronous cetuximab in patients with UICC stage III or high risk stage II (T2–3N0, T1–3N1) oropharyngeal, laryngeal (stage II glottic laryngeal excluded) or hypopharyngeal squamous cell carcinoma.
A planned 14 patients would be recruited and treated with 62.5 Gy in 25 daily fractions (dose level I) over five weeks with synchronous cetuximab. If none experienced grade 4 acute toxicity, a further 14 patients would be enrolled. The radiotherapy dose would then be escalated to 65 Gy in 26 daily fractions (dose level II). If more than two patients at a dose level experienced Grade 4 acute toxicity, then recruitment would be stopped. However, if one year after completion of recruitment to the first dose level of 62.5 Gy in 25 daily fractions there were no locoregional recurrences, then this cohort would be expanded to 28 patients in total and the second dose level of 65 Gy in 26 daily fractions not used. For oropharyngeal tumours, immunohistochemistry using the CINtec® p16INK4a assay was retrospectively performed on formalin-fixed paraffin- embedded (FFPE) tissue.
Patients gave written informed consent before enrolment. The study was registered with European Union Drug Regulating Authorities Clinical Trials (EudraCT number: 2007-000741-36) and approved by South Manchester Research Ethics Committee (REC number: 07/Q1403/32). The procedures followed were in accordance with the ethical standards of this committee and with the Helsinki Declaration of 1975, as revised in 1983.
Patient population
Inclusion criteria were: histologically proven oropharyngeal, laryngeal or hypopharyngeal squamous cell carcinoma; UICC stage II (oropharyngeal, supraglottic or subglottic laryngeal, hypopharyngeal) or III (T2–3N0, T1–3N1) disease; age ≥ 18 years; Eastern Cooperative Oncology Group (ECOG) performance status 0–2; adequate haematological, liver and renal function. Exclusion criteria were: stage II laryngeal glottic carcinoma, neck lymph nodes clinically or radiologically > 2 cm without neck dissection, any N2/N3 disease; prior surgical curative resection for primary tumour; patients with metastatic disease; prior radiotherapy within the treatment field; any relative contraindication to radiotherapy; prior administration of EGFR monoclonal antibodies, signal transduction inhibitors or targeted therapies; previous malignancy.
Treatment
Radiotherapy
All patients were immobilised with a custom made thermoplastic five-point fixation head, neck and shoulders shell. A planning CT scan was performed in the treatment position with a slice thickness of 3 mm. CT data were transferred to the Pinnacle Treatment Planning System (version 7.4f). The gross tumour volume (GTV) was defined as the primary tumour and involved lymph nodes, determined by clinical, endoscopic and imaging investigations. Involved lymph nodes were defined as those ≥ 10 mm in short axis diameter or presence of a necrotic centre. The clinical target volume (CTV) was the GTV and areas deemed at risk of microscopic disease. Three CTVs were delineated: CTV1, the GTV with an isotropic expansion of 10 mm (edited for natural barriers to disease spread including bone, air, fascia); CTV2, the remainder of the involved sub-site and nodal levels and uninvolved first echelon nodal levels; and CTV3, nodal levels deemed at lower risk of microscopic disease spread. Three planning target volumes (PTV1, PTV2, PTV3) were defined by an isotropic expansion of 3 mm from CTV1, CTV2 and CTV3, respectively.
Radiation doses to PTV1, PTV2 and PTV3 were 62.5 Gy, 55 Gy and 50 Gy over five weeks, respectively, prescribed to the mean of the PTV. The planning targets included that minimum and maximum doses to the PTV were within 95–105% of the prescription dose and less than 5% of the volume outside the PTV received > 105% of the prescription dose. IMRT was inversely planned and delivered using a simultaneous integrated boost technique, 6MV photons and a seven-field beam arrangement. Patients were treated in a supine position and positioned using markers on the thermoplastic shell and orthogonal laser beams. Treatment verification was performed using kilovoltage CT imaging for the first three treatment fractions and weekly thereafter. The verification action level was 3 mm.
Cetuximab
Cetuximab was administered intravenously once weekly commencing the week prior to radiotherapy and continuing during the treatment course. The first dose of cetuximab was 400 mg/m2; subsequent doses of 250 mg/m2 were administered 2–4 hours before radiotherapy. Pre-medications were intravenous chlorphenamine 10 mg and dexamethasone 8 mg. Patients were monitored for one hour following infusion for any adverse reactions.
Safety and late toxicity
Safety
Patients were evaluated for acute toxicity weekly during (weeks 0–6) and at 12 and 18 weeks after commencement of treatment. Assessments included measurement of weight and completion of physician-recorded toxicity questionnaires, based on the Common Terminology Criteria for Adverse Events (CTCAE version 3.0).
Cetuximab
For CTCAE grade ≥ 1 skin reactions, a combination of topical emollients, systemic antibiotics (e.g. oral doxycycline) and oral antihistamines were used. For CTCAE grade 3 skin toxicity, cetuximab was delayed for up to two consecutive infusions and resumed when this had resolved to CTCAE grade ≤ 2 without change in dose level. On the second and third occurrences of grade 3 skin toxicity, cetuximab was delayed for up to two consecutive weeks and when re-commenced was dose reduced to 200 mg/m2 and 150 mg/m2, respectively, without scope for subsequent increase. Cetuximab was discontinued if more than two consecutive infusions were withheld or on occurrence of a fourth grade 3 skin toxicity. In case of allergic/hypersensitivity reactions, treatment guidelines were followed and depending on severity the infusion rate was subsequently reduced or cetuximab discontinued.
Radiotherapy
For CTCAE grade ≤ 3 toxicities, supportive medications, such as analgesia and topical emollients, were prescribed. At the discretion of the treating oncologist or in the event of grade 4 toxicity, radiotherapy was interrupted until toxicity resolved to CTCAE grade ≤ 2. Compensation for radiotherapy treatment delays followed departmental policy in an attempt to ensure treatment did not exceed the intended overall treatment time.
Late toxicity
Objective and subjective late toxicity assessments were made at 6, 12, 18 and 24 months and then annually for a total of five years after completion of treatment using physician and patient completed questionnaires, which were based on CTCAE version 3.0. The impact of treatment on symptoms and quality of life was serially assessed by patient completed validated European Organisation for Research and Treatment of Cancer (EORTC) head and neck questionnaires (QLQ-C30 version 3 and QLQ-H&N35). These questionnaires were completed at baseline, 6, 12 and 18 weeks from commencement and 6, 12, 18, 24 months and then annually for a total of five years following completion of treatment.
Treatment effectiveness
All patients received a baseline CT or MRI scan. Patients were followed up six weekly for the first year, two monthly for the second year, three monthly for the third year, four monthly for the fourth year and then six monthly thereafter. Response was assessed six weeks following treatment by clinical examination of the tumour site and neck (direct visualisation orally and/or with a fibreoptic laryngoscope and by palpation); and at 12 weeks by radiological assessment with a CT or MRI scan. Subsequent imaging was performed as clinically indicated.
Statistical methods
The primary endpoint of this study was acute toxicity. Secondary endpoints were: late toxicity and quality of life; clinical response rate, loco-regional control, cause-specific survival and overall survival.
Primary analysis
The dose limiting toxicity (DLT) was defined as any grade 4 acute toxicity. The maximum tolerated dose (MTD) was the maximum radiotherapy dose that caused a DLT in ≤ 20% of patients. If more than two patients experienced a DLT at a dose level, recruitment would stop completely at that dose level. MTD would then be the preceding dose level. If one year after completion of recruitment to the first dose level of 62.5 Gy in 25 fractions, there were 0/14 loco-regional recurrences then this cohort would be expanded to a total of 28 patients and the second dose level of 65 Gy in 26 fractions not used.
Secondary analysis
Physician-recorded acute toxicity as well as physician- and patient-reported late toxicity were reported using descriptive statistics. Symptom and quality of life data were derived from validated EORTC QLQ-C30 version 3 and QLQ-H&N35 questionnaires. The scores were linearly converted on a scale of 0–100. A superior level of function was reflected as a high score for the global and function scales (e.g. physical or role) and a low score for the symptom scales (e.g. fatigue or pain). Comparisons between baseline and subsequent scores were made using: 1) descriptive statistics to determine subjective significance, where mean changes in score of 5–10 units are interpreted as ‘a little’, 10—20 units are ‘moderate’ and greater than 20 units ‘very much’ [Citation21]; and 2) Student's paired t-test, where a 99% confidence interval and a significance level of p < 0.01 was applied to account for multiple statistical comparisons between baseline and subsequent scores (comparisons between subsequent time points were not used due to small patient numbers). Overall cause-specific survival and overall survival were reported from the date of registration into the study and survival curves generated using the Kaplan-Meier survival analysis technique. Analyses were performed using SPSS version 20.0 and GraphPad Prism version 6.0.
Results
Between February 2008 and July 2009, 14 patients received radiation dose level I (62.5 Gy in 25 fractions). One patient in this cohort was excluded (during treatment) and replaced because the radiation treatment plan did not conform to the target doses for the lower risk organs at risk. Dose level II (65 Gy in 26 fractions) was not employed because there were no loco-regional recurrences or distant failures at one year. Between August 2010 and September 2011, a further 14 patients were enrolled to receive radiation dose level I (62.5 Gy in 25 fractions). One patient in this cohort was excluded for protocol violation. In total, 27 patients, 20 (74%) male and seven (26%) female of median age 63 years (range 41–75) were available for analysis. There were 17 (63%) oropharyngeal, eight (30%) laryngeal and two (7%) hypopharyngeal squamous cell carcinomas; six (22%) and 21 (78%) patients had UICC stage II and III disease, respectively. Only one (4%) patient underwent a selective neck dissection prior to radiotherapy. At the start of treatment, 48% of patients were current smokers. Human papilloma virus status was not a recognised prognostic factor at the time of study design. This was retrospectively determined for oropharyngeal tumours by p16 immunohistochemistry and positive in 10/16 evaluable cases. includes baseline patient and disease characteristics.
Table I. Baseline patient and disease characteristics.
Treatment compliance and safety
The radiotherapy course was completed in 26/27 patients; for one (4%) patient the final fraction was omitted due to skin toxicity. Of the remainder, 22 (85%) completed treatment as scheduled; the overall treatment time was extended by 1–3 days for four (15%) patients. This was respectively due to CTCAE grade 3 toxicity (pain/mucositis), an ill-fitting immobilisation mask (facial swelling), inability to lie flat for treatment (from abdominal pain following gastrostomy insertion) and inter- current infection (pneumonia). All six cycles of cetuximab were received by 23 (85%) patients. One (4%) patient discontinued cetuximab after three cycles due to derangement of liver function tests, three (11%) patients did not receive the final cycle of treatment due to grade 3 mucositis/dermatitis and in one (4%) case because of inter-current infection (pneumonia). The maximal acute toxicities assessed by CTCAE version 3.0 are reported by number of patients and percentage of total in . There were no CTCAE grade 4 acute toxicities. The proportions of patients experiencing CTCAE grade 3 acute toxicities were: pain (81%), oral mucositis (78%), dysphagia (41%), dermatitis (18%), nausea (11%) and acneiform rash (8%). Admission to hospital for supportive measures was required for 11 (41%) patients. A total of nine (33%) patients required nasogastric tube or gastrostomy feeding (1/9 prior to treatment). All physician-recorded toxicities, except in the case of one (4%) patient who developed grade 3 weight loss, improved between weeks 6, 12 and 18 from commencement of treatment (data not shown).
Table II. Maximum physician recorded acute toxicity (CTCAE version 3) during treatment (number of patients, percentage of total in parentheses).
Late toxicities
Maximal physician- and patient-reported late toxicities assessed using a questionnaire adapted from CTCAE version 3.0 and measured at 6, 12, 18 and 24 months and then annually for five years after completion of treatment are reported in and . The rates of physician-recorded severe late toxicities were low. There were no grade 4 and few grade 3 toxicities, which included: pain (11%), anorexia (8%), problems with teeth (8%) and weight loss (4%). At 12-months follow-up, only one (4%) patient required a feeding tube due to insertion prior to treatment for dysphagia. The maximal/peak rates of patient-reported late toxicities from six months included: severe pain (11%) that affected activities of daily living (15%); loss of appetite (44%); altered taste (78%); any dry mouth (89%) and ‘ropey’ saliva (59%); difficulty swallowing (70%) that required a soft/liquid diet (23%) or tube feeding (7%).
Table III. Maximum physician recorded late toxicity (based on CTCAE version 3.0) from 6 months after treatment (number of patients, percentage of total in parentheses).
Table IV. Patient-reported late toxicity (based on CTCAE version 3.0) from 6 months after treatment (number of patients and percentage of total).
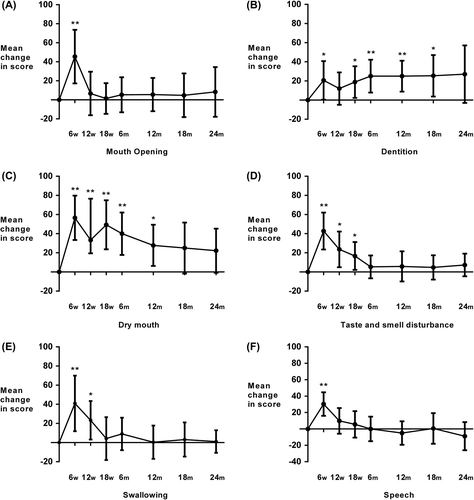
Compared with baseline, in the final week of radiotherapy (six weeks after commencement of treatment) most quality of life functional and symptom mean scores had ‘very much’ deteriorated (). However, 18 weeks after commencement of treatment most domains had recovered to only ‘a little’ or ‘moderately’ (role functioning, insomnia, less sexuality, taste and smell, loss of appetite, social eating, dentition) worse. Of note and as illustrated in , mean scores for swallowing dysfunction were only a little worse 18 weeks after commencement of treatment. Some parameters remained very much worse, which included social functioning, fatigue, dry mouth, sticky saliva, need for nutritional supplements and weight loss. At six months after treatment, of those moderately worse at 18 weeks only role function, less sexuality and loss of appetite remained in this category; the remainder had improved except for dentition, which deteriorated. However, all of the domains that were very much worse at 18 weeks persisted to six months except for fatigue, which showed improvement. At 12 months following treatment, all quality of life and most symptom domains mean scores had resolved to baseline levels (including swallowing dysfunction) or were only a little worse. The exceptions were need for nutritional supplements (moderately increased); and dry mouth, sticky saliva and dentition (very much worse). For these three domains this remained evident 24 months after completion of treatment. The only symptom domains statistically increased relative to baseline six months after treatment were need for nutritional supplements, dry mouth, sticky saliva and dentition scores (, p < 0.01). This persisted to 12 months for dry mouth; and 18 months follow-up for dentition and sticky saliva scores.
Figure 1. Patient-reported (EORTC QLQ–H&N35 questionnaire) change in mean symptoms scores from baseline at 6, 12 and 18 weeks from start of treatment and 6, 12, 18 and 24 months from completion of treamtment for A. mouth opening, B. dentition, C. dry mouth, D. taste and smell, D. swallowing, E.speech. Statistical comparisons are versus baseline scores, *p< 0.01, **p < 0.001; error bars represent 99% confidence intervals. w, weeks; m, months.
Effectiveness
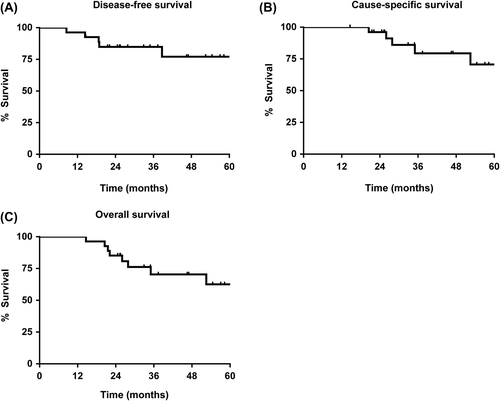
The median follow-up for surviving patients was 47 months (range 18–63 months). There was a 93% complete radiological response to treatment. One (4%) patient had a partial response but no evidence of disease on subsequent follow-up; and one (4%) patient with a pyriform fossa carcinoma (UICC stage III, T3N0) had radiological evidence of stable disease. Initial evaluation was negative for residual carcinoma but subsequently there was biopsy proven locally progressive disease. This was not amenable to surgical resection and the patient subsequently died of disease. Including this case, to date, there were a total of five loco-regional recurrences (median time to recurrence for these five patients was 18.7 months; range 8.5–38.7 months). Of these recurrences, three were oropharyngeal tonsil carcinomas (all UICC stage III, T2N1) and one an oropharyngeal posterior pharyngeal wall carcinoma (UICC stage III, T3N1). For 4/5 patients, the recurrences were within the 95% isodose for the high dose treatment volume (PTV1). There was one recurrence within the 95% isodose for the intermediate dose treatment volume (PTV2). Each of these patients subsequently died of disease. With a median follow-up of 47 months, the overall cause-specific survival was 79% (95% CI 53–92; five deaths), and the overall survival was 70% (95% CI 46–85; eight deaths). Kaplan-Meier plots for disease-free, cause-specific and overall survivals are shown in .
Discussion
This phase I/II study indicated that the use of a dose-intensified hypofractionated IMRT schedule (62.5 Gy in 25 daily fractions, 2.5 Gy per fraction) with synchronous cetuximab for intermediate stage HNSCC was: 1) safe, but at the limit of acute tolerability; 2) associated with low levels of late toxicity and impact on quality of life; and 3) effective, although this requires further evaluation.
The primary end point of this study was acute toxicity. There were no formal dose limiting toxicities (defined as occurrences of CTCAE grade 4 toxicities). However, the radiation schedule appeared to be at the limit of tolerability. This is in keeping with that predicted by the radiobiological BED modelling for acute mucosal reactions. The observed rate of CTCAE grade 3 mucositis was 78%; and hospitalisation required by 41% of patients. By comparison, in a systematic review of 33 studies including a total of 6181 patients, the mean rates of severe (grade 3/4) mucositis in those who received conventional fractionation radiotherapy alone, synchronous chemotherapy or altered fractionation schedules were 34%, 43% and 57%, respectively [Citation22]. Hospitalisation was reported in three of these studies and required for 21%, 14% and 66% of patients. Most (74%) patients experienced CTCAE grade 2 acneiform rash but there were relatively low incidences of grade 3 dermatitis (18%) and rash (8%).
The incidences of physician-recorded maximal severe CTCAE late toxicities were low. At 12-months follow-up, only one (4%) patient required a feeding tube, which was inserted prior to treatment for dysphagia. Patient-reported late toxicities and outcome measures demonstrated high incidences and persistence of both dry mouth and dental caries. The high rate of xerostomia correlates with the radiation doses achieved to the ipsilateral and contralateral parotid glands, which exceeded the mean target dose of 24 Gy in 78% and 67% of patients, respectively (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.958528). However, the observed late effects on swallowing, speech and mouth opening were less. Six months after treatment mean scores for swallowing dysfunction were only a little worse and by 12 months had returned to baseline. General domains of wellbeing including global health and functional scales also all returned to baseline levels 12 months after treatment. These results are in keeping with the finding that the impact of xerostomia is less pronounced than swallowing dysfunction on health- related quality of life [Citation6]. In the RTOG retrospective analysis of three synchronous chemotherapy non-IMRT trials (two employed conventional fractionation and one an accelerated concomitant boost schedule) including a total of 230 patients with locally advanced HNSCC, 43% developed severe late toxicity, including 27% swallowing dysfunction and 20% (29/142 assessable patients) long-term feeding tube dependence. However, this comparison should be interpreted with caution especially since bulk of disease (including tumour and nodal stage) is significantly associated with late morbidity [Citation5].
Altered fractionation regimens may be compared by use of the linear-quadratic equation to calculate the biologically equivalent dose (BED) [Citation23]. This correlates with tumour control, acute and late toxicities [Citation23]. The equivalent doses for conventionally fractionated treatments delivering 70 Gy in 35 daily fractions over seven weeks are: BED tumour 67.5 Gy10, log10 tumour cell kill 10.3, BED acute mucosa 53.1 Gy10 and BED late effects 116.7 Gy3. The corresponding doses for the investigational regimen of 62.5 Gy in 25 daily fractions over five weeks are: BED tumour 70.9 Gy10, log10 tumour cell kill 10.8, BED acute mucosa 58.3 Gy10 and BED late effects 114.6 Gy3.
There are concerns treatment acceleration with a maintained total radiation dose may result in unacceptable levels of acute toxicity and consequential late effects. In a trial that used 2D-planned radiotherapy, treatment to a total dose of 66 Gy with twice daily 2 Gy-fractions (BED acute mucosa 67.3 Gy10 and BED late effects 110 Gy3) resulted in unacceptable increases in grade 3 and 4 acute and late toxicities [Citation11]. In the continuous accelerated radiation (CAIR) trial, 66–72 Gy (74% of patients received 72 Gy) in 2 Gy-fractions delivered over five weeks (for 72 Gy, BED acute mucosa 64.2 Gy10 and BED late effects 120 Gy3) using 2D-planned radiotherapy was poorly tolerated, with significantly increased acute toxicity and consequential late soft tissue/bone necrosis seen in 22% of patients [Citation12]. These studies exceeded the estimated tolerance threshold for acute mucosal and pharyngeal reactions of 59–61 Gy10, [Citation24]. However, moderate acceleration with a shortening of overall treatment time by one week appears feasible and effective. In the DAnish Head And Neck CAncer (DAHANCA)-6&7 trial, an accelerated schedule of 66–68 Gy in 33–34 six weekly fractions (BED acute mucosa 55.4–57.0 Gy10 and BED late effects 110–113 Gy3) using a conventional technique improved locoregional control by 10% at five years; there were associated increases in some acute toxicities but these were reversible and there were no significant increases in late effects [Citation25].
It is axiomatic that with dose intensification/escalation there should be limitation to the irradiated volume [Citation20]. In the present study, this was achieved by careful selection of patients with lower disease burden and the use of IMRT and a geometric rather than anatomical approach to define smaller treatment volumes, a strategy that is not thought to increase the risk of local failure. In a recent early phase trial, 57 patients with T2N0 (61%), T2N1 (21%) and T3N0 (18%) oropharyngeal, laryngeal and hypopharyngeal HNSCC were treated with hypofractionated radiotherapy using a simultaneous integrated boost IMRT technique [Citation1]. The high dose volume received 69, 72 and 75 Gy in 30 fractions of 2.3, 2.4 and 2.5 Gy fractions, respectively over six weeks (BED tumour 73.0, 77.4, 81.9 Gy10; log10 tumour cell kill 11.1, 11.8, 12.4; BED acute mucosa 59.5, 63.9, 68.4 Gy10; BED late effects 121.9, 129.6, 137.5 Gy3, respectively). All patients completed treatment; the incidences of acute grade 3 and 4 toxicities were 56% and 5.3%. Late grade 3/4 toxicities were equally distributed between the groups and seen in 16% of all patients. While accepting the inherent weaknesses of cross-trial comparisons, our toxicity data are in keeping with these results.
With a median follow-up of 47 months, the overall cause-specific survival was 79% (95% CI 53–92). This is encouraging, especially in view of the high prevalence of active smoking (48%) in this cohort. We do not know the level of EGFR expression in this study, but it is of interest that data from the DAHANCA group suggest the benefit of radiotherapy acceleration is greatest in well-moderately differentiated tumours and those with high EGFR expression [Citation19].
Conclusion
Dose-intensified hypofractionated IMRT with 62.5 Gy in 25 daily fractions over five weeks and synchronous cetuximab for intermediate stage HNSCC is associated with acceptable levels of acute toxicity, low rates of late toxicity and impact on quality of life at 12 months following treatment. For patients with stage III disease it may represent an alternative schedule to spare the late morbidity associated with synchronous chemotherapy and radiotherapy. Further evaluation of effectiveness is recommended.
Supplementary material available online
Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.958528.
ionc_a_958528_sm8178.pdf
Download PDF (28.8 KB)Acknowledgements
The trial was sponsored by The Christie NHS Foundation Trust. Cetuximab was received complimentary of Merck Serono pharmaceuticals.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Leclerc M, Maingon P, Hamoir M, Dalban C, Calais G, Nuyts S, et al. A dose escalation study with intensity modulated radiation therapy (IMRT) in T2N0, T2N1, T3N0 squamous cell carcinomas (SCC) of the oropharynx, larynx and hypopharynx using a simultaneous integrated boost (SIB) approach. Radiother Oncol 2013;106:333–40.
- Wadsley JC, Bentzen SM. Investigation of relationship between change in locoregional control and change in overall survival in randomized controlled trials of modified radiotherapy in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004;60:1405–9.
- Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14.
- Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006;368:843–54.
- Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol 2008;26:3582–9.
- Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 2008;26:3770–6.
- Nguyen NP, Moltz CC, Frank C, Vos P, Smith HJ, Karlsson U, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol 2004;15:383–8.
- Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8.
- Ghi M, Paccagnella A, Ferrari D, Foa P, Rocca M, Verri E, et al. A phase II-III study comparing concomitant chemoradiotherapy (CRT) versus cetuximab/RT (CET/RT) with or without induction docetaxel/cisplatin/5-fluorouracil (TPF) in locally advanced head and neck squamous cell carcinoma (LASCCHN): Efficacy results (nct01086826). J Clin Oncol 2013;31(Suppl; abstr 6003).
- Riaz N, Sherman E, Koutcher L, Shapiro L, Katabi N, Zhang Z, et al. Concurrent chemoradiotherapy with cisplatin versus cetuximab for squamous cell carcinoma of the head and neck. Am J Clin Oncol Epub 2014 Jan 7.
- Jackson SM, Weir LM, Hay JH, Tsang VH, Durham JS. A randomised trial of accelerated versus conventional radiotherapy in head and neck cancer. Radiother Oncol 1997; 43:39–46.
- Skladowski K, Maciejewski B, Golen M, Pilecki B, Przeorek W, Tarnawski R. Randomized clinical trial on 7- day-continuous accelerated irradiation (CAIR) of head and neck cancer – report on 3-year tumour control and normal tissue toxicity. Radiother Oncol 2000;55:101–10.
- Dische S, Saunders M, Barrett A, Harvey A, Gibson D, Parmar M. A randomised multicentre trial of chart versus conventional radiotherapy in head and neck cancer. Radiother Oncol 1997;44:123–36.
- Fowler JF. Optimum overall times II: Extended modelling for head and neck radiotherapy. Clin Oncol (R Coll Radiol) 2008;20:113–26.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35.
- Bourhis J, Sire C, Graff P, Gregoire V, Maingon P, Calais G, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): An open-label phase 3 randomised trial. Lancet Oncol 2012;13:145–53.
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003;362:933–40.
- Baujat B, Bourhis J, Blanchard P, Overgaard J, Ang KK, Saunders M, et al. Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev 2010: CD002026.
- Eriksen JG, Steiniche T, Overgaard J. The influence of epidermal growth factor receptor and tumor differentiation on the response to accelerated radiotherapy of squamous cell carcinomas of the head and neck in the randomized DAHANCA 6 and 7 study. Radiother Oncol 2005;74:93–100.
- Fowler JF. Is there an optimum overall time for head and neck radiotherapy? A review, with new modelling. Clin Oncol (R Coll Radiol) 2007;19:8–22.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol 2003;66:253–62.
- Hartley A, Sanghera P, Kazi W, Mehanna H, McConkey C, Glaholm J, et al. Correlation of currently used radiobiological parameters with local control and acute and late mucosal toxicity in randomised studies of altered fractionation for locally advanced head and neck cancer. Clin Oncol (R Coll Radiol) 2011;23:29–33.
- Fowler JF, Harari PM, Leborgne F, Leborgne JH. Acute radiation reactions in oral and pharyngeal mucosa: Tolerable levels in altered fractionation schedules. Radiother Oncol 2003;69:161–8.
- Mortensen HR, Overgaard J, Specht L, Overgaard M, Johansen J, Evensen JF, et al. Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6 & 7 randomised trial with accelerated radiotherapy for head and neck cancer.Radiother Oncol 2012;103:69–75.